Abstract
Background
Pacemakers assist circulation by generating electrical impulses. Patients with pacemakers scheduled to undergo surgery are vulnerable to device-related complications. Therefore, careful perioperative management is required to prevent undesirable events.
Case
A 66-year-old man with alcohol-related hepatocellular carcinoma was referred for liver transplantation. The pacemaker was inserted preoperatively to manage sick sinus syndrome and paroxysmal atrial fibrillation. Overall liver transplantation was performed without any adverse events. However, the pacemaker suddenly failed to provide regular pacing rhythm during abdominal closure. Fortunately, the native heart rate was maintained above 70 beats per minute and blood pressure did not fluctuate after pacing failure. After retrospective analysis, the duration setting of preoperative pacemaker reprogramming (24 h) was revealed as the cause of unexpected pacing failure.
Go to : 
A pacemaker is a device that assists circulation by generating electrical impulses. An increasing number of arrhythmic patients are supported by pacemakers [1]. Pacemaker failure can be catastrophic for device-dependent patients. Patients with pacemakers or cardioverter/defibrillators who are scheduled to undergo surgery are vulnerable to device-related complications, including pacemaker malfunction. Cautious perioperative management, including magnet or reprogramming of the device to asynchronous mode, is required to prevent undesirable events [2]. Furthermore, the operation team should be aware of the current detailed status of pacemakers by reviewing medical documentation and cardiologist consultation. Here, we present the case of a patient with an unanticipated mode change of pacemaker during living donor liver transplantation. This case report was approved by our Institutional Review Board (no. 2022-11-075), and written informed consent was obtained from the patient for the publication of this case.
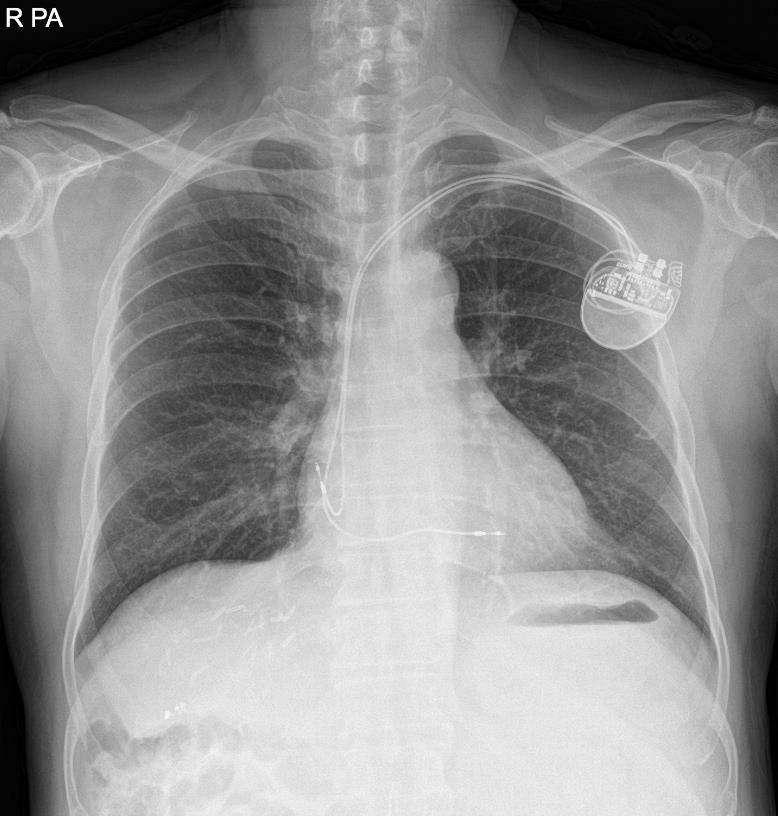
A 66-year-old, 82.4-kg, 167.5-cm man with alcohol-associated hepatocellular carcinoma underwent living-donor liver transplantation. He initially experienced intermittent chest discomfort after exercise one year before liver transplantation, and atrial fibrillation with a heart rate of 30 to 45 was observed on electrocardiogram. After short-term follow-up, he was diagnosed with sick sinus syndrome and paroxysmal atrial fibrillation with slow ventricular response or junctional bradycardia. A pacemaker (DDDR, ACCOLADE Magnetic Resonance Imaging [MRI] EL DR L331, Boston) was inserted one month before the surgery in order to manage arrhythmia (Fig. 1). Ventricular and atrial endocardial pacing leads were inserted into the left subclavian vein. They were positioned in the right ventricle midseptum and right atrium appendage using fluoroscopic guidance, respectively. He had no pacemaker-related problems since its application. Cardiologic consultation showed that the pacemaker was functioning well and tolerable for liver transplantation after its mode change.
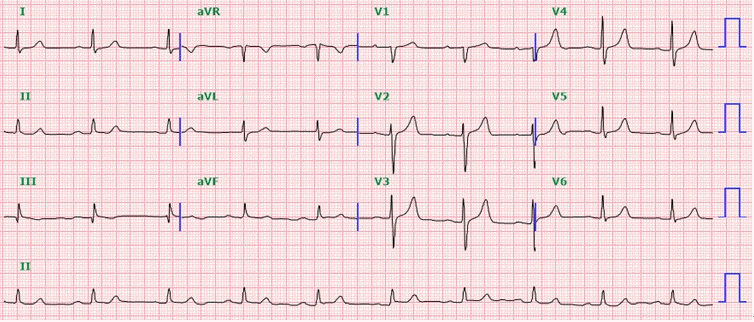
The initial laboratory results were 11.0 g/dl hemoglobin, 35.4% hematocrit, 118 × 103/μl platelet count, 14.0 s prothrombin time with an international normalized ratio of 1.10, and 38.5 s activated partial thromboplastin time. Preoperative electrocardiogram showed sinus bradycardia with a rate of 58 beats per minute and first degree atrioventricular block (Fig. 2). Diastolic dysfunction grade 1 with left atrial enlargement and minimal tricuspid regurgitation was found on echocardiography. The left ventricular ejection fraction was 70%, and no regional wall motion abnormalities were observed. The preoperative interrogation report showed that his pacemaker was programmed for synchronous dual-chamber pacing (DDD) at 50–130 beats per min. The patient’s intrinsic rhythm was a sinus with 60 beats per min. The sensitivity of the pacemaker electrode is defined as the minimum myocardial voltage required to be detected as a P-wave or R-wave. The original state of atrial sensitivity was 0.75 mV, and right ventricular sensitivity was 2.5 mV. The pacemaker output was the current delivered in a burst from the pulse generator. The initial atrial output was 3.5 V per 0.40 ms, and right ventricular output was 3.5 V per 0.40 ms.
The pacemaker mode was reprogrammed preoperatively. Since the pacemaker was capable of functioning during MRI scanning, a cardiac implantable electronic device (CIED) specialist selected MRI mode for reprogramming and protection mode time out; the time limitation that pacemaker mode automatically reverses to the original status was set to 24 h. Unfortunately, the anesthesiologist and surgeon were unaware of the detailed information on pacemaker interrogation. The pre-anesthetic visit notes did not include details pertaining to the pacemaker model, variables, and settings.
After standard monitoring including a 5-lead electrocardiogram, general anesthesia was induced with intravenous thiopental sodium 350 mg, vecuronium 8 mg and sevoflurane. Intubation was performed easily with a plain endotracheal tube. The patient was mechanically ventilated with a tidal volume of 6 ml/kg and a positive end-expiratory pressure of 6 cmH2O. Invasive arterial blood pressure monitoring was performed with right radial artery and right femoral artery cannulation. The right femoral vein was catheterized to monitor compression of the inferior vena cava during the surgical procedure. A 9-Fr catheter (ARROW MAC 2 lumen, Teleflex) and pulmonary artery catheter (Swan-ganz CCOmbo V, Edwards Lifesciences) were inserted via the right internal jugular vein. Anesthesia was maintained with sevoflurane and continuous infusion of vecuronium (0.6 μg/kg/min). Intravenous dopamine (3–10 μg/kg/min) and norepinephrine (0.05–0.1 μg/kg/min) were continuously infused to maintain mean blood pressure above 70 mmHg. Intravenous dopamine (5 μg/kg/min) was administered during the reperfusion period. Epinephrine was not required during the whole period of liver transplantation, including the reperfusion period. Blood loss, calculated as lost red cell mass, was 1,341 ml [3] and total urine output was 770 ml. One unit of pre-leukocyte-reduced red blood cells, two units of fresh frozen plasma and 963 ml of autologous red cell mass acquired from Cell Saver was transfused during transplantation. The total anesthesia time was 8 h and 59 min.
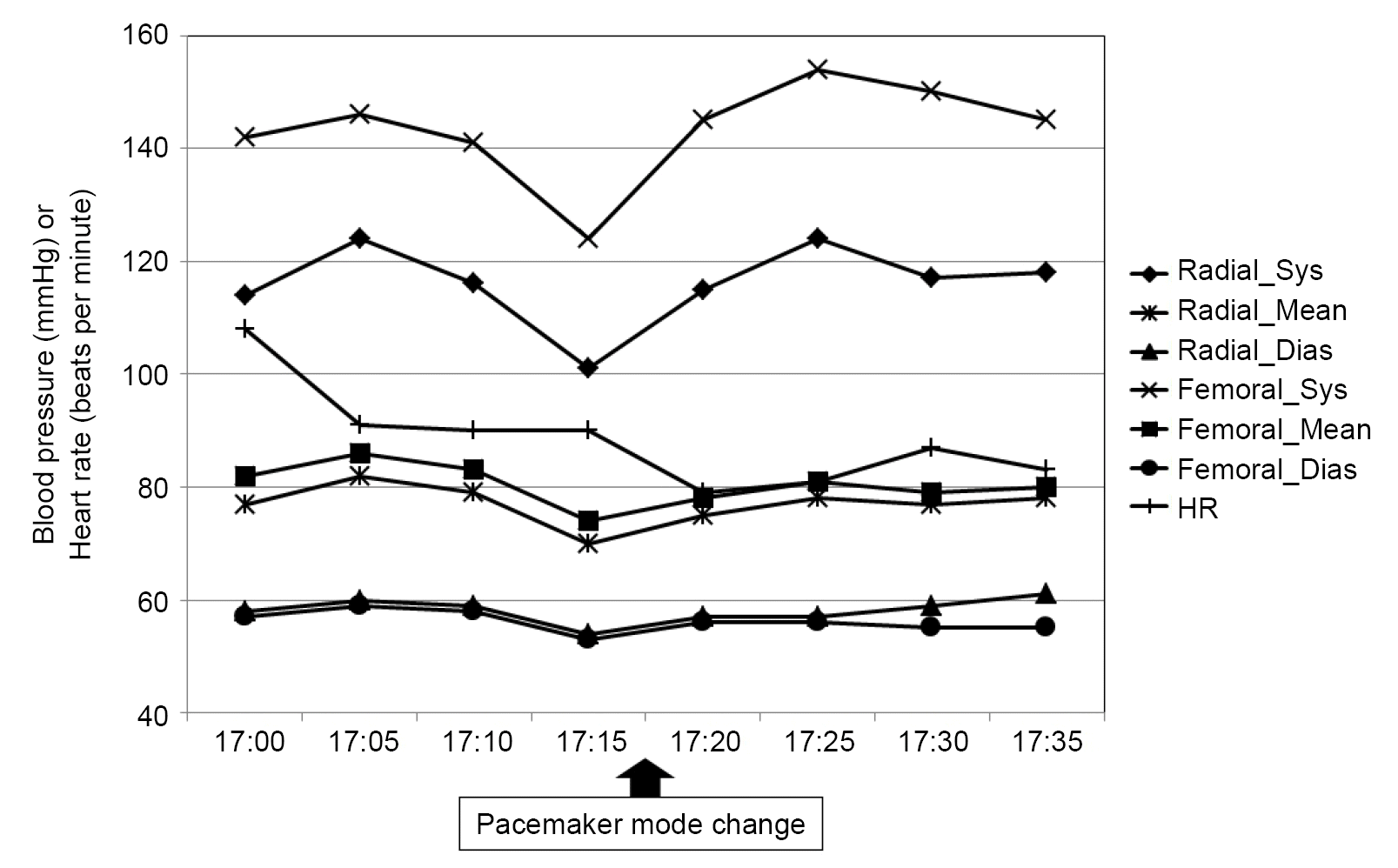
Overall, liver transplantation was performed without adverse events. The pacing rhythm was maintained at 90 bpm. However, the pacemaker suddenly failed to provide a regular pacing rhythm during abdominal closure with staplers. The heart rate slowed at 70–80 bpm, and QT prolongation was observed on electrocardiography. ST-II was –0.3 and ST-V was –0.5 at the time of the event, and there was no abnormality in the PR interval. Arterial blood gas analysis showed normokalemia (K+ 5.3 mmol/L) and mild acidosis (pH 7.278, pCO2 42.4 mmHg, HCO3- 19.3 mmol/L, base excess –7.0 mmol/L). No additional antiarrhythmic drugs were infused during the operation. In summary, pacemaker malfunction was not likely caused by metabolic abnormalities or myocardial ischemia. Surgeons were immediately notified of pacing errors. There was no probable surgical procedure that might have deteriorated the pacemaker function. Intravenous atropine 0.25 mg was administered to prevent further delay in cardiac rhythm. CIED specialists were not available immediately after the unexpected pacemaker mode changes as it occurred outside their regular working hours. Fortunately, the native heart rate was maintained above 70 bpm, and the blood pressure did not fluctuate after pacing failure (Fig. 3). The risk of electromagnetic interference (EMI) was low because the additional use of monopolar electrocautery was not expected for residual operations. Since the operation was about to end, we decided to transfer the patient to the intensive care unit without additional medical management or interrogation of the pacemaker function. Moreover, on-call CIED specialists were urgently consulted for additional pacemaker interrogation because the attending anesthesiologists suspected a pacemaker mode change.
The patient was transferred to the intensive care unit (ICU) postoperatively with a continuous infusion of dopamine 10 μg/kg/min and norepinephrine 0.05 μg/kg/min. Norepinephrine was discontinued immediately after arrival due to high blood pressure, and intravenous remifentanil infusion was started for sedation. Dopamine was tapered off the day after surgery. The postoperative laboratory findings were as follows: hemoglobin 10.2 g/dl, hematocrit 33.2%, platelet count 98×103/µl, prothrombin time 28.6 s with an international normalized ratio 2.81, and activated partial thromboplastin time 83.7 s. Pacemaker interrogation was performed by a cardiologist and on-call CIED specialist 24 min after arrival at the ICU, and the pacemaker was found to be in DDD mode, which implies automatic preset reversal. There were no technical issues regarding the pacemaker battery or lead position changes. Upon retrospective analysis, the preoperative pacemaker reprogramming duration setting of 24 h was revealed to be the cause of unexpected pacing failure. The pacemaker mode was reprogrammed from DDD to DOO with 90 beats per min, on the evening of the day before surgery by a CIED specialist to prevent EMI caused by intraoperative use of electrocautery. Both atrial and right ventricular outputs were converted to 5.0 V per 1.0 ms. Additional information on pacemaker interrogation is shown in Table 1. The CIED specialist did not consider 24 h to be insufficient for liver transplantation and reprogrammed the pacemaker using a routine preoperative setting that was appropriate for short and simple surgical procedures. Postoperatively, the anesthesiologists provided feedback, including preoperative confirmation of pacemaker mode change and cooperation flow to handle emergent pacemaker crises, to the CIED specialist to prevent recurrent events.
Timeline of Pacemaker Interrogation (Liver transplantation [22.06.21 08:37-17:36])
The following day, the patient was referred for emergent surgical bleeding control. His pacemaker was rearranged to asynchronous mode (DOO mode) just before the operation to prevent repetitive errors (Table 1). During the second operation, a 4 × 4 cm hematoma was found in the subhepatic hilar portion. No active bleeding was found after hematoma evacuation, and additional ligation with bleeding control was performed. Five days after liver transplantation, the patient was transferred to the general ward with stable vital signs. On the day of the follow-up outpatient appointment, the anesthesiologist visited the patient and explained the cause of the intraoperative event and further possibilities for pacemaker mode change.
Go to : 
Cardiac pacemakers have rapidly evolved from simple electrical stimulators to advanced medical devices that can provide personalized anti-arrhythmic treatment. The first modern cardiac pacemaker was developed in 1932 by American physiologist Albert S. Hyman, who created an electromechanical device powered by a hand-cranked motor. Hyman attempted to provide electric impulses to the patient’s right atrium by connecting an external direct current generator and bipolar needle electrode inserted via an intercostal space [4,5]. The first totally implantable internal pacemaker was introduced by Elmqvist and Senning in 1958. It was implanted in a 43-year-old engineer named Arne Larsson who had suffered from a complete heart block [4]. The development of cardiac pacemakers was followed by detailed programming including rate responsiveness and multichamber pacing. Emerging techniques are focused on remote monitoring, leadless, and batteryless devices [6]. The pacemaker inserted in this patient was a dual-chamber pacemaker with rate responsivity, which is safe in MRI environments. Specifically, the pacemaker can be reprogrammed to AOO, VOO, or DOO modes, representing asynchronous atrial, ventricular, and dual pacing, respectively, prior to MRI scanning. MRI time-out mode can be used to automatically return the patient to the original pacemaker setting after the scan, and the duration can be customized depending on the type of procedure: off, 12, 24, and 48 h [7].
Although hemodynamic instability was not observed in this case, it could have led to catastrophic events considering the long surgical duration of liver transplantation. Preoperatively, preoperative assessment, including direct inquiry into any symptoms related to device malfunction, should be performed. Electrolyte abnormalities, acid-base disturbances, and blood gas analysis should be evaluated because they may influence pacemaker function. Lead fractures or migration may be identified using chest radiography. Recent electrocardiography should be performed, and spikes preceding all P-waves and the QRS complex may indicate pacemaker dependency [8]. Reprogramming of the cardiac pacemaker should be considered if the patient is highly pacemaker-dependent or if advanced functions such as rate responsivity or sleep/rest mode are in use [8].
A main shortcoming of the present case was that the duration of preoperative pacemaker reprogramming was too short and should have been evaluated to determine whether it was long enough until the end of liver transplantation. An unexpected pacemaker mode reversal to the original DDD setting can increase the risk of EMI. Common consequences of EMI are inappropriate inhibition or triggering of pacemaker signals, and reversal to asynchronous pacing. In particular, EMI oversensing by the atrial channel of the pacemaker in DDD mode can result in pacemaker-mediated tachycardia.
To prevent potential interference, bipolar electrical diathermy is considered safer than monopolar diathermy. If monopolar diathermy is required, it should be used in cutting rather than coagulation mode, and limited to 1–2 bursts after 10-s pauses. If EMI is likely to occur, the pacemaker should be set to asynchronous mode (ex. VOO, DOO) by reprogramming the device or using a magnet [2,8]. The major advantages of magnets are that they are reversible, easily available, and do not require specialists. However, the indiscriminate application of magnets can cause potential hazards [9]. The magnet cannot switch pacemaker mode if it is positioned incorrectly. Magnet may not alter pacemaker mode in patients who are obese or have abdominal or submuscular implants. In addition, magnet behavior can be unpredictable when applied to pacemakers with low battery voltage. To prevent fatal intraoperative arrhythmias, equipment should be available for external defibrillation and temporary pacing. If it is difficult to approach the chest wall during the procedure, an external patch should be attached before surgery. Defibrillator pads should be placed at least 10–15 cm away from the pacemaker to prevent damaging of the device. Postoperatively, the patient’s cardiac rate and rhythm should be monitored continuously, and pacemaker function should be immediately interrogated [2].
If a pacemaker fails to pace the ventricle, several causes of pacemaker malfunction should be considered. In recent devices, generator and lead failures are relatively rare. Additional conditions, such as acid-base or electrolyte imbalances, myocardial ischemia, or elevated plasma concentrations of antiarrhythmic drugs may contribute as possible causes [10]. In this case, acid-base and electrolyte imbalances were ruled out by performing arterial blood gas analysis. Additionally, the pacing threshold usually increases in cases of pacemaker malfunction caused by myocardial ischemia [11]. Myocardial ischemia can be confirmed using coronary angiography.
To manage this situation, the patient’s escape rate and cardiac rhythm should be analyzed. If the rate is slow, intravenous atropine 0.5 mg or isoproterenol infusion (1–2 μg/min) may be considered as medical treatment [12]. Cardiopulmonary resuscitation and advanced cardiac life support are not contraindicated when hemodynamic instability occurs because of pacemaker malfunction. Defibrillation or cardioversion can be performed, but the current may damage the pacing and sensing circuits of the pacemaker. To minimize this risk, the defibrillation paddle should be located as far as possible from the generator. Additionally, external pacing should be considered for possible pacing failure after defibrillation or cardioversion [13].
Anesthesiologists should be alert when treating patients with implantable pacemakers because minor errors may lead to inadvertent pacing failure or severe hemodynamic instability. In particular, the hemodynamics of cirrhotic patients should be monitored cautiously because increased arrhythmic risk and subsequent cardiovascular symptoms have been reported, including chronotropic incompetence, cardiomyopathy, prolonged QT intervals, hyperdynamic circulation, and impaired ventricular contractility [14]. As hemodynamic instability during liver transplantation is an independent predictor of recipient mortality and graft failure [15], cautious preoperative and intraoperative management is required to prevent unexpected pacing failure and surgical complications.
Go to : 
Notes
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
AUTHOR CONTRIBUTIONS
Conceptualization: Gaabsoo Kim. Methodology: Sooyeon Lee. Writing - original draft: Sooyeon Lee. Writing - review & editing: Gaabsoo Kim, Sooyeon Lee. Investigation: Sooyeon Lee. Supervision: Gaabsoo Kim.
Go to : 
REFERENCES
1. Bae MH. Cardiac implantable electronic device therapy in Korea: increasing but still quite low. Korean Circ J. 2019; 49:853–5.

2. Practice advisory for the perioperative management of patients with cardiac implantable electronic devices: pacemakers and implantable cardioverter-defibrillators 2020: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with cardiac implantable electronic devices: erratum. Anesthesiology 2020; 132: 938. Erratum for: Anesthesiology. 2020; 132:225–52.
3. Bang SR, Ahn HJ, Kim GS, Yang M, Gwak MS, Ko JS, et al. Predictors of high intraoperative blood loss derived by simple and objective method in adult living donor liver transplantation. Transplant Proc. 2010; 42:4148–50.

4. Mulpuru SK, Madhavan M, McLeod CJ, Cha YM, Friedman PA. Cardiac pacemakers: function, troubleshooting, and management: part 1 of a 2-part series. J Am Coll Cardiol. 2017; 69:189–210.
5. Aquilina O. A brief history of cardiac pacing. Images Paediatr Cardiol. 2006; 8:17–81.
6. Madhavan M, Mulpuru SK, McLeod CJ, Cha YM, Friedman PA. Advances and future directions in cardiac pacemakers: part 2 of a 2-part series. J Am Coll Cardiol. 2017; 69:211–35.
7. Boston Scientific. ACCOLADE MRI pacemaker spec sheet. Scientific [Internet]. 2016 [updated 2020; cited 2022 Oct 20]. Available from https://www.bostonscientific.com/content/dam/bostonscientific/ep/general/CRM-324101-AE_ACCOLADEMRI%20Pacemaker%20SpecSheet-FINAL.pdf.
8. Stone ME, Salter B, Fischer A. Perioperative management of patients with cardiac implantable electronic devices. Br J Anaesth. 2011; 107 Suppl 1:i16–26.

9. Schulman PM, Rozner MA. Case report: use caution when applying magnets to pacemakers or defibrillators for surgery. Anesth Analg. 2013; 117:422–7.
10. Kitajima A, Nakatomi T, Otsuka Y, Sanui M, Lefor AK. Cardiac arrest due to failed pacemaker capture after peripheral nerve blockade with levobupivacaine: a case report. A A Pract. 2021; 15:e01445.

11. Umei TC, Awaya T, Okazaki O, Hara H, Hiroi Y. Pacemaker malfunction after acute myocardial infarction in a patient with wrap-around left anterior descending artery supplying the right ventricular apex. J Cardiol Cases. 2018; 18:9–12.

12. Bready LL, Dillman D, Noorily SH. Decision making in anesthesiology. 4th ed. Philadelphia (PA);Mosby: 2007.
13. Lam C. Permanent cardiac pacemaker: an emergency perspective. Hong Kong J Emerg Med. 2001; 8:169–75.

15. De Maria S Jr, Nürnberg J, Lin HM, Contreras-Saldivar AG, Levin M, Flax K, et al. Association of intraoperative blood pressure instability with adverse outcomes after liver transplantation. Minerva Anestesiol. 2013; 79:604–16.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download