INTRODUCTION
Various hypnotics are used for general anesthesia in many surgeries including laparoscopic surgery. It is very important for an anesthesiologist to select the most ideal drug considering the patient and surgical characteristics. In particular, in laparoscopic surgery where pneumoperitoneum occurs, starting and maintaining anesthesia with an intravenous anesthetic showed better advantages than inhalational anesthesia [
1]. Propofol is currently the most commonly used intravenous anesthetic. It has a rapid onset of action, a short half-life and is associated with rapid recovery of cognitive ability [
2-
4].
However, propofol can lead to injection pain, propofol infusion syndrome, and hemodynamic and respiratory depression [
4,
5]. Midazolam is associated with relatively less cardiovascular and respiratory depression than propofol [
6]. However, it has a slower onset of action and active metabolites can cause prolonged recovery time. Therefore, it is relatively difficult to control the depth of anesthesia with midazolam [
7]. Therefore, there is a need for new anesthetic drugs with high efficacy and fewer side effects while providing stable and controllable anesthesia.
Remimazolam, a newly developed anesthetic, is an ultra-short-acting intravenous benzodiazepine with a high affinity for the benzodiazepine binding site of the γ-Aminobutyric acid type A (GABA-A) receptor. It is rapidly hydrolyzed to an inactive metabolite by non-specific tissue esterases. These properties allow for faster and more predictable recovery after sedation with remimazolam compared to other benzodiazepines, such as midazolam [
8]. Moreover, it has the hemodynamically stable properties of benzodiazepines and the rapid onset-offset properties of propofol [
8-
12]. Since remimazolam was recently approved as a general anesthetic, many studies have demonstrated the efficacy and safety of remimazolam during induction and maintenance of general anesthesia [
13-
17]. However, recovery after the end of general anesthesia is important for the patient's prognosis. There have been few studies on recovery from anesthesia after using remimazolam for general anesthesia induction and maintenance. Therefore, this study aimed to compare recovery profiles after intravenous anesthesia with remimazolam and propofol in patients undergoing laparoscopic cholecystectomy.
Go to :

MATERIALS AND METHODS
This prospective, single-center, randomized, single-blind, controlled study was approved by the Institutional Review Board of our hospital (DAUHIRB-21-211) and registered at the Korea Clinical Research Information Service (permit number: 0006702). The trial was conducted at the our hospital between November 2021 and March 2022, in accordance with the Declaration of Helsinki. All patients provided informed consent before enrollment to the study.
The inclusion criteria were as follows: age 20–80 years, American Society of Anesthesiologists physical status I–III, scheduled for elective laparoscopic cholecystectomy, and provided informed consent. The exclusion criteria were as follows: ASA physical status IV or V, body mass index ≥ 35 kg/m2, expected difficult airway intubation, history of surgery, and refusal to provide informed consent.
Patients were randomly distributed into a remimazolam or propofol group using computer-generated randomization prior to surgery. An anesthesiologist explained the purpose of the study and the possible side effects to the patient and obtained informed consent for the induction of anesthesia before surgery.
Routine monitoring, including electrocardiogram, non-invasive blood pressure (BP), pulse oxygen saturation, and bispectral index (BIS), were performed upon the arrival of each patient in the operating room. During this time, to ensure that the patient is blinded to the procedure, the arm that was to be injected with the drug during anesthesia induction was abducted by 90°, and a shield was temporarily placed to cover the arm, using a surgical cloth.
Before the induction of general anesthesia, oxygen (flow rate, 10 L/min) was administered through a mask for 2 min to all patients in the supine position. After preoxygenation, target-controlled infusion of remifentanil with an effect-site concentration (Ce) of 4.0 ng/ml was initiated. Moreover, remimazolam was simultaneously administered to induce anesthesia with a bolus of 0.2 mg/kg slowly within 1 min [
13,
14]. In the control group, 2.0 mg/kg propofol was administered as a bolus. Pain was assessed during drug injection. The time to loss of consciousness (LOC) was recorded. LOC was defined as a Modified Observer’s Assessment of Alertness/Sedation score < 2 [
11]. After confirming LOC, neuromuscular blockade was performed with 0.8 mg/kg rocuronium. An i-gel (Intersurgical) was inserted when the train-of-four (TOF) count was zero. At the same time, the shield used for blinding was removed and a drug infusion line using a syringe pump was connected to the patient to maintain anesthesia.
The respiratory settings were as follows: inspired fresh gas at 3 L/min through the circle anesthesia breathing system, inspired tidal volume preset at 9 ml/kg, and inspiratory/expiratory ratio of 1:2. The respiratory rate was adjusted to maintain the end-tidal carbon dioxide values at 30–40 cmH2O.
Initially, Remimazolam at 1.5 mg/kg/h and propofol at 8 mg/kg/h were administered to each group to maintain anesthesia. The Ce of remifentanil was 2.5 ng/ml. During surgery, systolic BP (SBP), diastolic BP (DBP), heart rate (HR), and BIS were monitored and recorded in both groups at baseline(immediately after entering the operating room), before intubation, 1 min after intubation, 5 min before CO2 pneumoperitoneum, 5 min after the start of CO2 pneumoperitoneum, 5 min before pneumoperitoneum removal, and 5 min after pneumoperitoneum removal (T0–T6, respectively).
Doses were adjusted according to changes in hemodynamics or BIS. When BIS was > 60 or < 40, remimazolam and propofol were adjusted within the range of 1-2 mg/kg/h and 4-12 mg/kg/h, respectively. When the SBP decreased by 20% of that initially and was maintained for 1 min, the Ce of remifentanil was reduced by 0.5 ng/ml. However, if the SBP increased by 20% of that initially and was maintained for 1 min, the Ce of remifentanil was increased by 0.5 ng/ml. Phenylephrine (intravenous [IV], 100 μg) was administered if hypotension was detected (SBP < 90 mmHg, despite reducing remifentanil). Atropine (0.5 mg IV) was administered when bradycardia (HR < 50) occurred. The frequency of vasopressor or atropine administration was recorded.
All drug infusions were terminated at the end of surgery. Sugammadex was administered to counteract the effects of rocuronium as follows: if the TOF count was 0, 4 mg/kg sugammadex was administered; if the TOF count was 1–4, 2 mg/kg sugammadex was administered.
After terminating drug infusion, recovery from anesthesia was evaluated by checking the time to eye opening, spontaneous breathing, extubation, orientation recovery (Orientation check asks the patient for their name), requirement for analgesics in the recovery room, and the time to complete recovery (Aldrete score of 9). A modified Brice interview [
18] was conducted to evaluate awareness during the operation.
The primary outcome was complete recovery. The secondary outcomes were (i) the time to LOC (time to MOAA/S < 2); (ii) hemodynamic changes during maintenance of anesthesia; (iii) time to opening eyes, spontaneous breathing, and extubation after discontinuation of drug infusion; and (ⅳ) time to recovery of orientation.
In a pilot study of 40 patients (20 patients in each group), the time to an Aldrete score of 9 was 42.5 ± 9.8 min in the remimazolam group and 37.1 ± 9.0 min in the propofol group. A sample size of 49 patients in each group was calculated with a type I error of 0.05 and power of 80%. Considering a 10% loss to follow-up, 54 patients were required per group.
Statistical analyses were performed using SPSS Statistics Version 18.0 (IBM Co.). Data are expressed as mean ± standard deviation and number of patients (%). Student’s t-test was used to analyze continuous data and the chi-squared or Fisher’s exact test were used to analyze categorical data. Statistical significance was set at P < 0.05.
Go to :

RESULTS
We recruited 111 patients to the study, but 3 patients refused to participate. Thus, 108 patients were included in this study. However, seven patients were excluded from the analysis: one patient in the remimazolam group and three in the propofol group because laparoscopic cholecystectomy was changed to open cholecystectomy during surgery, and two patients in the remimazolam group and one patient in the propofol group underwent endotracheal tube intubation. Finally, the data of 101 patients were evaluated (
Fig. 1).
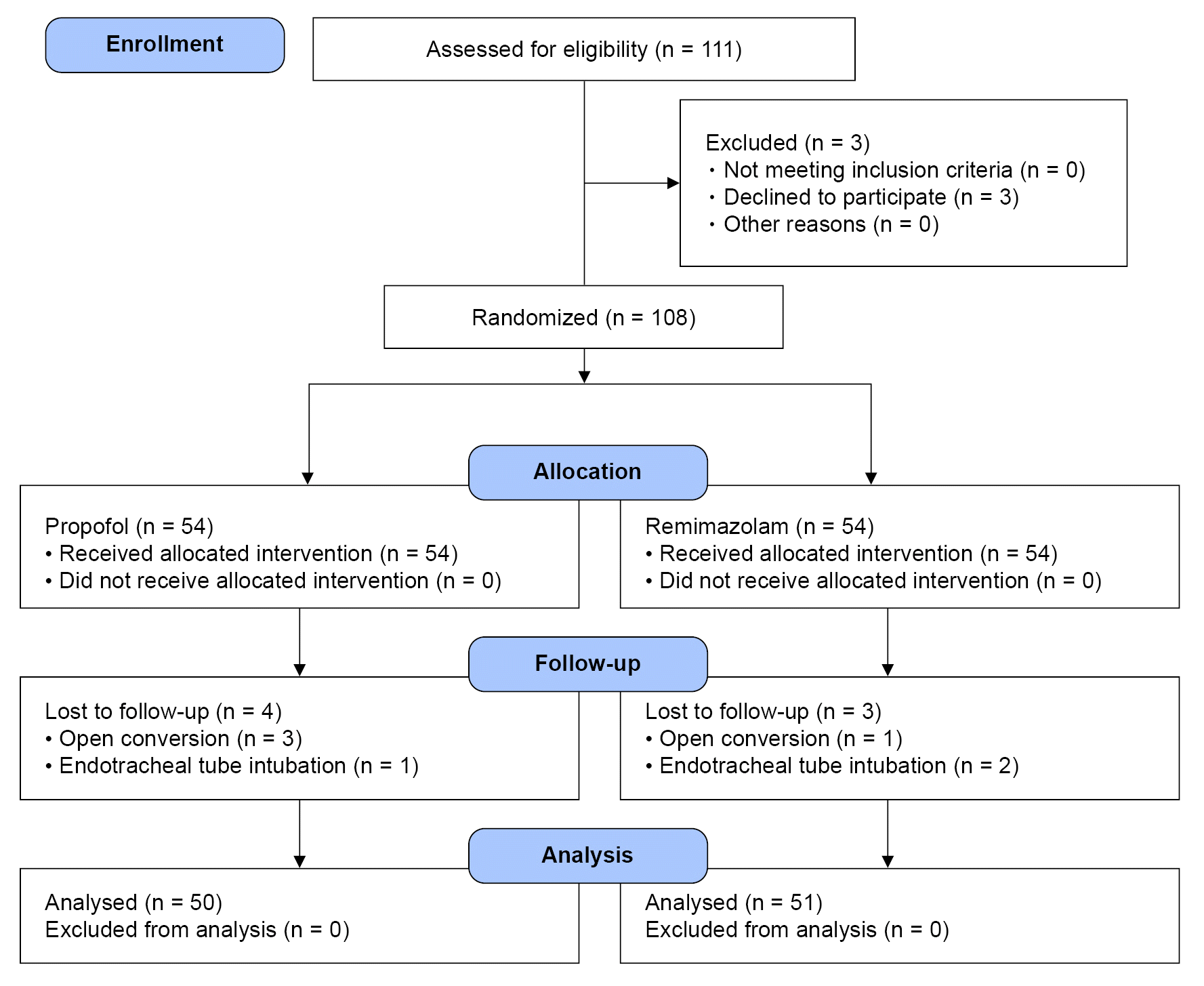 | Fig. 1.CONSORT flow diagram for patient enrollment. CONSORT: consolidated standards of reporting trials. 
|
There were no differences in demographic or surgical data between groups (
Table 1). The anesthesia induction and recovery times are presented in
Table 2. Complete recovery time (22.72 ± 7.06 vs. 25.75 ± 6.84 min, P = 0.031), as assessed by Aldrete’s score, was longer in the remimazolam group than in the propofol group. There was no difference in the LOC time between groups. The time to eye opening, recovery orientation, and requirement for analgesics in the recovery room were longer in the remimazolam group than in the propofol group. The time to return to spontaneous breathing and extubation time were not significantly different between groups.
Table 1.
Demographic and Surgical Data
|
Variable |
Propofol (n = 50) |
Remimazolam (n = 51) |
P value |
|
Age (yr) |
55.8 ± 15.8 |
51.6 ± 14.2 |
0.161 |
|
Sex (M/F) |
19/31 |
18/33 |
0.778 |
|
Height (cm) |
162.5 ± 9.4 |
161.6 ± 8.2 |
0.595 |
|
Weight (kg) |
65.9 ± 11.3 |
63.1 ± 10.8 |
0.215 |
|
BMI (kg/m²) |
24.9 ± 3.2 |
24.2 ± 3.6 |
0.298 |
|
ASA (I/II/III) |
15/24/11 |
18/25/8 |
0.685 |
|
Duration of surgery (min) |
18.3 ± 9.7 |
18.7 ± 10.7 |
0.865 |
|
Anesthetic time (min) |
55.4 ± 11.6 |
58.5 ± 15.8 |
0.256 |

Table 2.
Induction and Recovery Times
|
Variable |
Propofol (n = 50) |
Remimazolam (n = 51) |
P value |
Median (95% CI) |
|
Loss of consciousness (s) |
33.4 ± 6.3 |
35.8 ± 9.6 |
0.139 |
2.404 (–0.795 to 5.603) |
|
Eyes open (min) |
8.9 ± 3.6 |
10.6 ± 3.7 |
0.018 |
1.748 (0.307 to 3.189) |
|
Spontaneous breathing (min) |
9.4 ± 3.3 |
9.8 ± 3.1 |
0.524 |
0.405 (–0.852 to 1.661) |
|
Extubation (min) |
9.7 ± 3.3 |
10.1 ± 3.2 |
0.585 |
0.359 (–0.941 to 1.659) |
|
Orientation recovery (min) |
14.6 ± 5.3 |
19.5 ± 5.7 |
< 0.001 |
4.811 (2.621 to 7.001) |
|
Analgesic request (min) |
19.2 ± 6.1 |
22.8 ± 7.4 |
0.028 |
3.56 (0.872 to 6.248) |
|
Complete recovery (min) |
22.7 ± 7.1 |
25.7 ± 6.8 |
0.031 |
3.025 (0.282 to 5.768) |

Injection pain was significantly higher during drug injection in the propofol group than in the remimazolam group (98% vs. 1.9%, P < 0.001). HR was significantly higher in the remimazolam group than in the propofol group at T1 (73.5 ± 13.4 vs. 82.6 ± 14.9 bpm, P = 0.002), T2 (71.9 ± 14.3 vs. 80.6 ± 15.8 bpm, P = 0.005), T4 (77.5 ± 13.2 vs. 83.1 ± 14.7 bpm, P = 0.048), T5 (76.9 ± 12.4 vs. 83.6 ± 15.1 bpm, P = 0.016), and T6 (73.9 ± 11.7 vs. 80.1 ± 12.2 bpm, P = 0.010) (
Fig. 2). Moreover, there was no difference in the frequency of atropine administered between groups (
Table 3). There were no significant differences between groups regarding changes in SBP or DBP (
Fig. 2). However, the frequency of administration of vasopressor was lower in the remimazolam group than in the propofol group (
Table 3). The BIS was higher in the remimazolam group than in the propofol group at all measured timepoints (P < 0.001) (
Fig. 3). No patient developed awareness while maintaining anesthesia in either group.
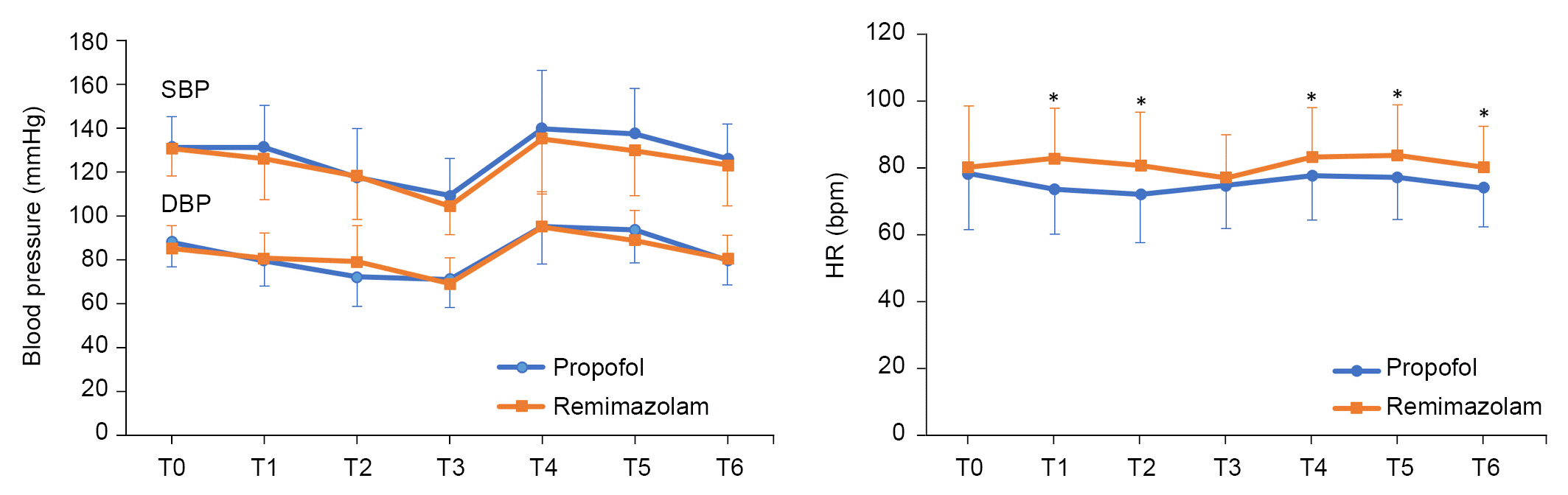 | Fig. 2.Changes in systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR). Baseline (T0), before intubation (T1), 1 min after intubation (T2), 5 min before CO2 pneumoperitoneum (T3), 5 min after the start of CO2 pneumoperitoneum (T4), 5 min before pneumoperitoneum removal (T5), and 5 min after pneumoperitoneum removal (T6). *P < 0.05 indicates a significant difference from the propofol group. 
|
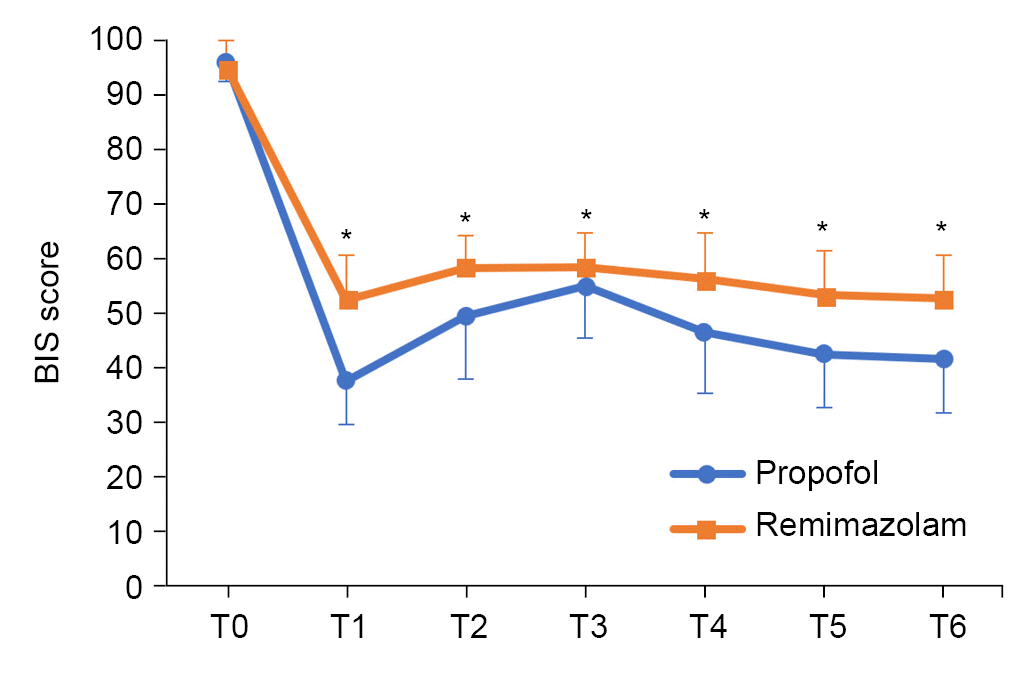 | Fig. 3.Changes in bispectral index (BIS). Baseline (T0), before intubation (T1), 1 min after intubation (T2), 5 min before CO2 pneumoperitoneum (T3), 5 min after the start of CO2 pneumoperitoneum (T4), 5 min before pneumoperitoneum removal (T5), and 5 min after pneumoperitoneum removal (T6). *P < 0.05 indicates a significant difference from the propofol group. 
|
Table 3.
Medications during Maintenance of General Anesthesia
|
Medication |
Propofol (n = 50) |
Remimazolam (n = 51) |
P value |
|
Total propofol (mg/kg) |
5.3 ± 1.7 |
- |
- |
|
Total remimazolam (mg/kg) |
- |
1.1 ± 0.3 |
- |
|
Total remifentanil (µg/kg) |
5.3 ± 1.5 |
6.0 ± 2.1 |
0.141 |
|
Phenylephrine (n) |
18 (36.0) |
9 (17.6) |
0.045 |
|
Atropine (n) |
9 (18.0) |
3 (5.9) |
0.072 |

Go to :

DISCUSSION
Remimazolam is a novel, ultra-short-acting benzodiazepine. It acts mainly on GABA-A receptors and is advantageous due to fast induction, fast recovery, and hemodynamic stability. It is currently being used for several clinical procedural sedation and anesthetic procedures, such as induction and maintenance of general anesthesia, hysteroscopy, endoscopy, and bronchoscopy [
16,
17]. However, studies on its clinical application in laparoscopic surgery are lacking. This study compared remimazolam and propofol, focusing on the recovery profile after general anesthesia, and also investigated the effects of anesthesia induction and intraoperative hemodynamic changes in patients undergoing laparoscopic cholecystectomy.
As of now, there are no TCI models integrated into commercially available infusion pumps for administering remimazolam. Therefore, we anesthetized participants by administering a single bolus of remimazolam in the same manner as propofol during anesthesia induction [
14]. The average time to LOC after bolus administration of remimazolam was similar to that after propofol administration, and no serious events occurred. Our results indicate that a single injection of remimazolam shows equivalent effects of propofol in terms of efficacy for induction. However, additional research is needed on the efficacy and safety according to the concentration of remimazolam when administered as a bolus.
Patients undergoing laparoscopic surgery experience hemodynamic changes during pneumoperitoneum. Intravenous anesthesia is advantageous due to hemodynamic stability and faster recovery than with inhalation anesthesia in laparoscopic surgery. Propofol is a commonly administered intravenous anesthetic with rapid induction and recovery. However, it decreases systemic BP due to a reduced cardiac output [
4].
In this study, SBP and DBP were not significantly different between groups. However, the frequency of vasopressor use was higher in the propofol group than in the remimazolam group. Therefore, the BP increased by vasopressor administration may be reflected at each timepoint in the propofol group, although without statistical significance. Although HR was higher in the remimazolam group, each value was within the clinically normal range. In our study, there was no case of additional administration of atropine for bradycardia after phenylephrine bolus administration. However, phenylephrine is a pure vasopressor that only has activity on the alpha-adrenergic receptors. Because it does not have any beta agonist properties to support the cardiac output, activation of the baroreceptor may result in bradycardia.
It is important to monitor the depth of general anesthesia. The BIS is a safe and effective method for monitoring the depth of anesthesia during surgery [
19]. Intense anesthesia can cause hemodynamic changes, whereas too-shallow anesthesia risks recall or awareness during anesthesia. Awareness during anesthesia is a serious complication with potential psychological sequelae, such as anxiety and posttraumatic disorders. Thus, the BIS value is generally maintained between 40 and 60 to prevent awareness during general anesthesia [
20].
In our study, BIS was higher during induction and maintenance of anesthesia in the remimazolam group than in the propofol group. The BIS of remimazolam was often close to 60 (
Fig. 3) and even exceeded 70 in some cases.
A modified Brice interview [
18] of patients was conducted within 24 h after the end of anesthesia. No patient in either group developed awareness while maintaining anesthesia.
However, even if there were no patients with intraoperative recall based on the questionnaire, it is possible that sedation was insufficient because the BIS remained high in the remimazolam group. Moreover, to adjust the BIS between 40 and 60, the frequency of dose increase was higher in the remimazolam group, which may have resulted in a longer recovery time.
Therefore, during anesthesia with remimazolam, it is necessary to use supportive indicators as well as BIS to assess the depth of sedation [
21].
In the present study, flumazenil was not administered for comparison with propofol after discontinuation of drug infusion. Complete recovery time, eye opening, orientation recovery and analgesic requirement were longer in the remimazolam group than in the propofol group. In a previous study, flumazenil was routinely administered to patients in the remimazolam group immediately after the completion of anesthesia, and the same dose of saline was administered to those in the propofol group. Recovery of consciousness, extubation, and postanesthetic care unit stay times were shorter in the remimazolam group than in the propofol group [
13]. Therefore, rapid recovery from anesthesia can be expected by administering flumazenil to patients in the remimazolam group.
This study have limitation. First, for LOC during anesthesia induction, we administered 0.2 mg/kg of remimazolam as a single bolus to all age groups. However, older adults may require a lower dose of anesthetic than younger or middle-aged patients due to the physiological changes associated with aging. Therefore, it is necessary to adjust the dose of remimazolam according to age. An other study has shown that the 95% effective dose (ED
95) of remimazolam bolus required to reach LOC during induction of anesthesia varies with age. The dose of remimazolam for induction of anesthesia was lower in the elderly group ≥ 60 years of age than in the younger group < 60 years [
22].
In conclusion, remimazolam provides safe induction and maintenance of anesthesia, and may be an effective alternative to propofol. Patients in the remimazolam group took longer to completely recover from anesthesia. Additionally, remimazolam may provide hemodynamic stability and satisfactory anesthetic effects in patients undergoing laparoscopic cholecystectomy. Nevertheless, large-cohort multicenter studies are warranted to validate the findings.
Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download