Abstract
Background
Common regional anesthesia approaches for video-assisted thoracoscopic surgery (VATS) include paravertebral block (PVB) and erector spinae plane block (ESPB). PVB is considered a deep nerve block which is contraindicated in antithrombotic therapy. ESPB is effective when administered as a bolus, as well as continuously. However, the recently proposed intertransverse process block (ITPB) ensures more effective diffusion of the local anesthetic into the paravertebral space.
Case
We report cases of three patients who received bolus ITPB (costotransverse foramen block and mid-point transverse process-to-pleura block in one and two cases, respectively) combined with continuous ESPB when a deep nerve block could not be administered. Opioids were not required postoperatively, and all postoperative numerical rating scale scores (0–10) at rest were maintained below 4.
Video-assisted thoracoscopic surgery (VATS) is a minimally invasive procedure with reduced surgical stress and postoperative pain compared with open thoracotomy. However, it is associated with significant acute pain regardless of wound size [1]. Paravertebral block (PVB), erector spinae plane block (ESPB), serratus anterior plane block, and intercostal block have been proposed as analgesic techniques for VATS [2,3]. PVB is strongly recommended, but is often contraindicated in some patients, such as those receiving antithrombotic therapy. ESPB has recently garnered attention owing to its proven non-inferior analgesic efficacy compared to PVB and its safety profile in reducing the occurrence of pneumothorax [4]. However, the mechanism underlying the action of ESPB remains unclear. In particular, the diffusion of local anesthetics into the paravertebral space is uncertain when the ESPB approach is used [5].
Moreover, the mid-point transverse process-to-pleura block (MTPB) and costotransverse foramen block (CTFB) have been proposed [6,7]. They are conceptually classified as intertransverse process blocks (ITPB) by the American Society of Regional Anesthesia and Pain Medicine (ASRA) and European Society of Regional Anaesthesia and Pain Therapy (ESRA) consensus on standardizing nomenclature [8]. ITPB is expected to be more effective than ESPB in ensuring a more reliable local anesthetic diffusion into the paravertebral space. Reports of its efficacy have been rising; however, data on catheter placement and continuous administration of local anesthetics are limited, with only a few reports on MTPB [9]. The space behind the superior costotransverse ligament, which is the target site of MTPB, is surrounded by the intertransverse ligament and muscles, fatty tissue, and the superior costotransverse ligament [5]. During the continuous administration of local anesthetics via MTPB, fixation of the inserted catheter into this space and the stability of its effect are uncertain. Continuous administration of local anesthetics through ESPB has been well studied, and catheter placement along the fascial surface is expected to provide stability. ESPB is classified as a superficial nerve block with few contraindications [10].
In cases of VATS, in which a deep nerve block is contraindicated, ESPB may be considered as an alternative; however, ITPB may be more effective than ESPB through bolus administration. Therefore, we combined the bolus ITPB and continuous ESPB in our report. Here, we report a case series of anesthetic management during VATS using a combination of bolus ITPB and continuous ESPB. We have submitted the consent forms for these three patients to the Editorial office, so there are no discrepancies.
A 72-year-old man (164 cm, 58.1 kg) with a history of diabetic neuropathy was diagnosed with lung cancer during a medical checkup. He was scheduled to undergo thoracoscopic pulmonary lobectomy with four ports (two each in the fifth and eighth intercostal spaces). Neuraxial anesthesia and PVB were avoided to negate the effects of the nerve block if neurosensory abnormalities were exacerbated postoperatively; therefore, we considered general anesthesia combined with bolus CTFB and continuous ESPB.
Rapid induction was achieved using 0.2 μg/kg/min of remifentanil, 40 mg of propofol, and 50 mg of rocuronium, which were added after establishing peripheral intravenous access. After induction of general anesthesia and placement of the patient in the right lateral position, a puncture at the Th5–6 level, which was the location of the main port, was created under ultrasound guidance. In addition, saline solution was injected to check the needle tip. After confirming the needle tip position, 20 ml of 0.25% levobupivacaine was injected as CTFB (Fig. 1). Color Doppler was used to continuously check the position of the needle tip while the local anesthetic was injected. Subsequently, a catheter was inserted into the erector spinae plane at the same vertebral level (Fig. 2), an infuser pump (COOPDECH Balloonjector Medical Co., LTD; 0.17% levobupivacaine, 4 ml/h; bolus, 4 ml; lockout time, 60 min) was connected, and postoperative continuous analgesia was initiated. The patient’s hemodynamics remained stable during the surgery with the administration of 4% desflurane and remifentanil (0.03 μg/kg/min). Operating and anesthetic times were 186 min and 269 min, respectively. The patient received 300 μg of intravenous fentanyl (100 µg immediately before the surgery, 100 µg at the time of wound closure, and 100 µg added during the surgery at the discretion of the anesthesiologist in charge) and 1,000 mg of acetaminophen intraoperatively.
Postoperative pain was measured using an 11-point numerical rating scale (NRS; 0, no pain; 10, worst pain imaginable). The patient’s postsurgical NRS scores at 2 h, 24 h, and 48 h were 4, 2, and 0 at rest, respectively. The NRS scores at 24 h and 48 h postoperatively were 3 and 5, respectively, upon movement. A postoperative pinprick test revealed an effective area from the parasternal to the anterior axillary line up to Th3–6.
The postoperative pain did not worsen after block termination. The ESPB catheter was removed 42 h postoperatively, and the patient was discharged from the hospital on postoperative day (POD) 6.
A 35-year-old man (164 cm, 79.6 kg) with right pulmonary apex pneumothorax from lung fistula formation due to nontuberculous mycobacteriosis was scheduled for fistula closure with three-port VATS (one and two in the sixth and eighth intercostal spaces, respectively). He refused epidural anesthesia because of the fear induced by his first surgery; therefore, he was managed perioperatively with general anesthesia and intravenous patient-controlled analgesia (IV-PCA) for the first time. Nausea occurred frequently with IV-PCA. During the second fistula closure, general anesthesia was induced and a single dose of ESPB (0.25% levobupivacaine, 30 ml, at the Th5–6 level) was administered to reduce opioid consumption; however, postoperative nausea still occurred due to the IV-PCA connection. For the third instance, we decided to add a continuous nerve block. Rapid induction was achieved using 0.2 μg/kg/min of remifentanil, 140 mg of propofol, and 50 mg of rocuronium, which were added after establishing peripheral intravenous access. Anesthesia was maintained with 6% desflurane and remifentanil (0.08–0.20 μg/kg/min). After induction of general anesthesia and placement of the patient in the left lateral position, MTPB was administered with 15-ml bolus of 0.25% levobupivacaine at the Th5–6 and Th7–8 levels. The needle tip was checked using the method described in case 1, and the catheter was placed in the space widened with normal saline beneath the erector spinae plane of Th7–8, where the thoracic drain would be placed postoperatively. The anesthesiologist in charge decided to increase the remifentanil dosage to 0.2 μg/kg/min because of the additional intraoperative dissection of the seventh rib and a 10-cm skin incision; however, the patient’s vital signs indicated that opioid might have been sufficient. Continuous ESPB (0.17% levobupivacaine, 4 ml/h) was administered postoperatively. The operating and anesthetic times were 132 min and 252 min, respectively. The patient received 300 μg of intravenous fentanyl (100 µg immediately before the surgery, 100 µg at the time of wound closure, and 100 µg added during the surgery at the discretion of the anesthesiologist in charge) and 1,000 mg of acetaminophen intraoperatively. The postoperative NRS scores at 2 h, 24 h, and 48 h were 0, 1, and 0, respectively, at rest and without a bolus requirement. The NRS score after movement at 24 h and 48 h postoperatively was 5 for both. The NRS was audible; however, the pinprick test was not performed because of the wound dressing on the 10-cm skin incision. Postoperative nausea was not observed, and the catheter was removed on POD 2.
A 70-year-old man (168 cm, 72.4 kg) with right upper lobe lung cancer was scheduled to undergo right upper lobectomy with four-port VATS (two each in the fourth and seventh intercostal spaces). He had undergone percutaneous coronary intervention on the left anterior descending and circumflex branches 8 years prior because of angina pectoris with other complications such as diabetes, stroke, chronic atrial fibrillation, chronic renal failure, and spinal canal stenosis. Antithrombotic therapy with apixaban and clopidogrel was withdrawn 2 days and 14 days, respectively, preoperatively. Although neuraxial block or PVB was not absolutely contraindicated, they were not performed because of the risk of hematoma and the patient’s early postoperative anticoagulation schedule. Rapid induction was achieved using 0.25 μg/kg/min of remifentanil, 100 μg of fentanyl, 40 mg of propofol, and 70 mg of rocuronium, which were added after establishing peripheral intravenous access. Anesthesia was maintained with 1.3% sevoflurane and remifentanil (0.11–0.18 μg/kg/min). After induction of general anesthesia and placement of the patient in the left lateral position, MTPB was administered with a 20-ml bolus of 0.25% levobupivacaine at the Th5–6 and Th7–8 levels. The placement of the needle tip and catheter was checked using the method mentioned in case 2. Continuous ESPB (0.17% levobupivacaine, 4 ml/h) was administered postoperatively. Operating and anesthetic times were 150 min and 237 min, respectively. The patient received 150 μg of intravenous fentanyl (100 μg at the time of anesthesia induction and 50 μg at the time of wound closure) and 1,000 mg of acetaminophen intraoperatively. His postoperative NRS scores at 2 h, 24 h, and 48 h were 0, 3, and 0, respectively, at rest and without a bolus requirement. A postoperative pinprick test revealed an effective area at Th4–8 of the anterior axillary line. The NRS scores after movement at 24 h and 48 h postoperatively were 8 and 5, respectively. The ESPB catheter was removed on the afternoon of POD 1 to resume the antithrombotic therapy.
VATS is a minimally invasive surgical option for patients with lung malignancy or pneumothorax. However, it often requires multiple intercostal port holes, which cause unbearable postoperative pain. Guidelines for enhanced recovery recommend epidural anesthesia and PVB as postoperative analgesia techniques for lung surgeries, such as VATS and open thoracotomy [2]. However, epidural anesthesia is ineffective in preventing chronic pain and adverse events, including urinary retention, hypotension, and muscle weakness, whereas PVB is used in VATS for procedure-specific postoperative management, as recommended by the ESRA [3].
PVB must be avoided as epidural anesthesia in patients undergoing antithrombotic therapy [10]. Although PVB is highly recommended, performing it in high-risk patients may not be advisable because of its high failure rate (10%) [11] and possible complications, such as pneumothorax. The analgesic management strategy in VATS for procedure-specific postoperative pain management proposed by ESRA recommends ESPB as grade A for a single shot and grade B for continuous administration [3]. Thus, ESPB is considered a good alternative to PVB; however, its mechanism of action remains unclear. Some proposed mechanisms of action include analgesic effects mediated by elevated local anesthetic plasma concentrations due to systemic absorption, nerve innervation of the thoracolumbar fascia, and immunomodulatory analgesic effects through the lymphatic system [5]. Whether ESPB induces blockade through the direct spread of local anesthetics to the paravertebral space remains controversial.
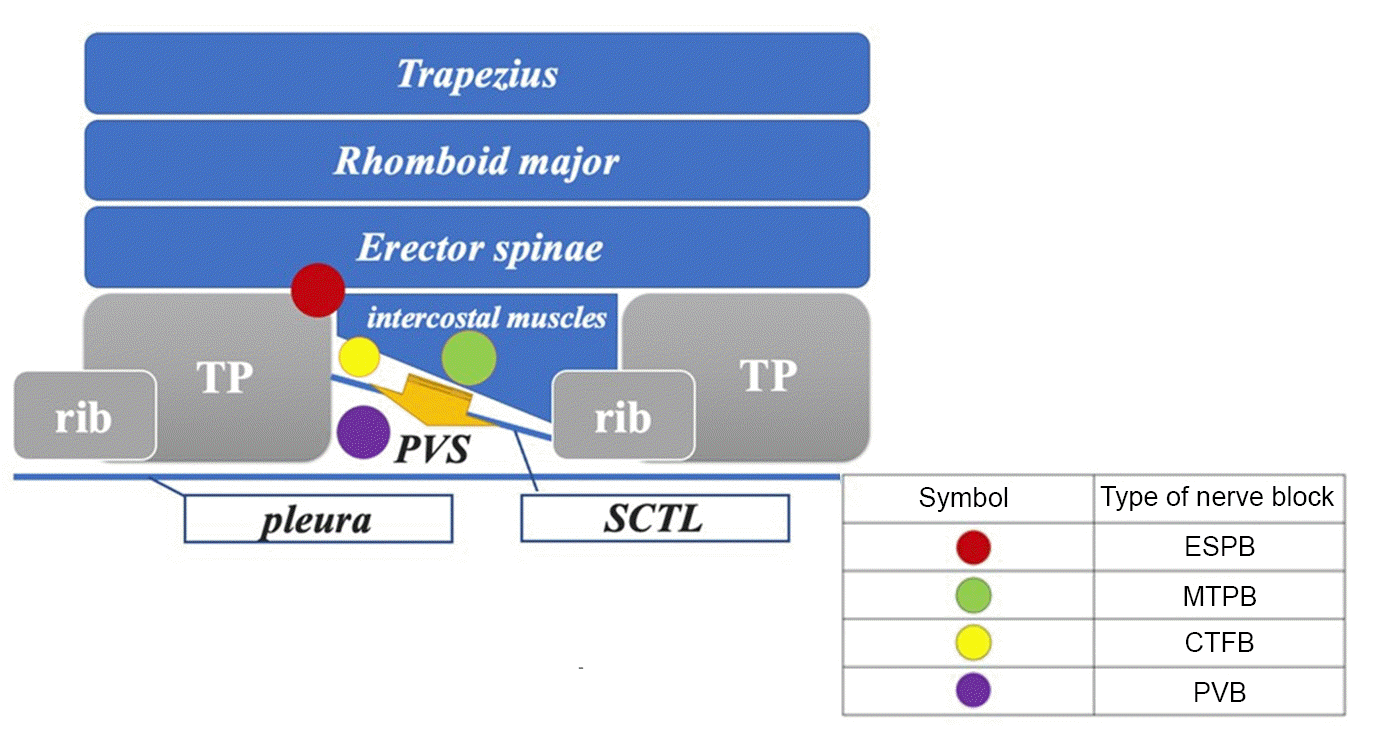
ITPB is considered more effective than ESPB because it involves the administration of a local anesthetic into a space deeper than the erector spinae plane and shallower than the superior costotransverse ligament, which forms the posterior part of the paravertebral space. MTPB and CTFB techniques were first reported in 2017 and 2020, respectively [6,7]. Although these blocks differ in terms of local anesthetic administration, they are conceptually classified as ITPB by the ASRA/ESRA nomenclature [8]. In the single-injection technique, the superiority of ESPB and ITPB has not yet been studied; however, when the diffusion pathway is considered, ITPB may be more effective because local anesthetics diffuse more reliably into the paravertebral space (Fig. 3) [5]. Therefore, we chose ITPB for single-injection blocks.
The single-injection block approach was tailored for each case. In case 1, CTFB was selected because the patient was relatively thin and CTFB images could be obtained easily. Shibata et al. [7] also reported the use of CTFB in two female patients weighing 48–50 kg. Conversely, MTPB was selected for overweight patients with body mass indices > 25. Although image rendition may largely depend on the operator’s skill and performance of ultrasound equipment, the influence of body size on the effect of CTFB and MTPB remains unclear. For single-shot MTPB, the time to first opioid demand was 12 h for VATS [12], and for single-shot CTFB, the duration of the blockade was 6–8 h [7]. Therefore, additional analgesia methods were considered necessary to obtain analgesia overnight. Considering the side effects of opioids, a continuous peripheral nerve block is preferred over IV-PCA.
Catheter insertion for the continuous administration of anesthetics with MTPB is difficult [9], and the actual fixation of the catheter in the retro-superior costotransverse ligament space and the stability of its effect are uncertain. In contrast, in a cadaveric study on ESPB, the diffusion of local anesthetics into the paravertebral space was controversial [13]. However, the following factors suggest the possibility of diffusion into the paravertebral space in a living human: (a) the posterior wall of the paravertebral space is slit-like and not completely closed by the superior costotransverse ligament [14], (b) pleural negative pressure due to breathing, and (c) erector spinae muscle contraction.
Although there are concerns regarding hematoma formation with continuous ESPB with catheter placement, the latest guidelines classify ESPB as a superficial nerve block [10]. Therefore, we inserted a catheter into the erector spinae plane and administered continuous ESPB. In fact, in the present cases, including case 3, apparent hematoma or findings suggestive of a hematoma were not observed.
The best way to continuously administer ESPB is debatable. Intermittent mechanical dosing methods are typically effective; however, continuous ESPB dosing using disposable balloon injectors has also been reported [15]. As a superior method of administration has not yet been established, continuous ESPB with a balloon injector, to which we were accustomed, was selected. Opioids were not required postoperatively, and a combination of peripheral nerve blocks, acetaminophen, and nonsteroidal anti-inflammatory drugs were used to manage postoperative analgesia.
In summary, when a deep nerve block such as PVB cannot be administered, ESPB may be considered as an alternative. There have been negative reports on ESPB regarding its originally proposed mechanism of action: the diffusion of local anesthetics into the paravertebral space. MTPB and CTFB, which were classified under ITPB, seem to be more reliable than ESPB in this aspect. However, evidence of ITPB in terms of analgesia with catheter placement and continuous administration has been lacking. Thus, we considered the clinical evidence for continuous ESPB and the safety of ESPB as a superficial nerve block. This report is significant as it shows that bolus ITPB and continuous ESPB may be superior to a combination of single-dose ESPB and continuous ESPB, as an alternative to PVB. To our knowledge, this is the first report on the combined use of bolus ITPB and continuous ESPB in VATS. These cases support the combined use as an effective postoperative analgesia strategy in cases where deep nerve blocks, such as PVB, cannot be used in VATS.
Notes
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
AUTHOR CONTRIBUTIONS
Conceptualization: Nobuhiro Tanaka. Data curation: Yuki Yamamoto, Yuma Kadoya, Miki Umehara, Takanori Suzuka. Visualization: Takanori Suzuka. Writing - original draft: Yuki Yamamoto, Nobuhiro Tanaka. Writing - review & editing: Nobuhiro Tanaka, Yuma Kadoya, Miki Umehara, Takanori Suzuka, Masahiko Kawaguchi. Supervision: Masahiko Kawaguchi.
REFERENCES
1. Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016; 17:836–44.

2. Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019; 55:91–115.

3. Feray S, Lubach J, Joshi GP, Bonnet F, Van de Velde M; PROSPECT Working Group *of the European Society of Regional Anaesthesia and Pain Therapy. PROSPECT guidelines for video-assisted thoracoscopic surgery: a systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2022; 77:311–25.

4. Taketa Y, Irisawa Y, Fujitani T. Comparison of ultrasound-guided erector spinae plane block and thoracic paravertebral block for postoperative analgesia after video-assisted thoracic surgery: a randomized controlled non-inferiority clinical trial. Reg Anesth Pain Med. 2020; 45:10–5.

5. Kim SH. Anatomical classification and clinical application of thoracic paraspinal blocks. Korean J Anesthesiol. 2022; 75:295–306.

6. Costache I, de Neumann L, Ramnanan CJ, Goodwin SL, Pawa A, Abdallah FW, et al. The mid-point transverse process to pleura (MTP) block: a new end-point for thoracic paravertebral block. Anaesthesia. 2017; 72:1230–6.

7. Shibata Y, Kampitak W, Tansatit T. The novel costotransverse foramen block technique: distribution characteristics of injectate compared with erector spinae plane block. Pain Physician. 2020; 23:E305–14.
8. El-Boghdadly K, Wolmarans M, Stengel AD, Albrecht E, Chin KJ, Elsharkawy H, et al. Standardizing nomenclature in regional anesthesia: an ASRA-ESRA Delphi consensus study of abdominal wall, paraspinal, and chest wall blocks. Reg Anesth Pain Med. 2021; 46:571–80.

9. Swathi KB, Kamal M, Kumar M, Kumar R, Chhabra S, Bhatia P. Comparison of analgesic efficacy of the conventional approach and mid-transverse process to pleura approach of the paravertebral block in video-assisted thoracoscopy surgeries: a randomised controlled trial. Indian J Anaesth. 2021; 65:512–8.

10. Kietaibl S, Ferrandis R, Godier A, Llau J, Lobo C, Macfarlane AJ, et al. Regional anaesthesia in patients on antithrombotic drugs: joint ESAIC/ESRA guidelines. Eur J Anaesthesiol. 2022; 39:100–32.
11. Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth 2006; 96: 418-26. Erratum in: Br J Anaesth. 2007; 99:768.
12. Chen X, Yang J, Xia M, Wu H, Wang S, Zhang W. Single-injection midpoint transverse process-to- pleura block versus thoracic paravertebral block for postoperative analgesia after uniportal video-assisted thoracoscopic surgery: a randomized controlled trial. J Cardiothorac Vasc Anesth. 2022; 36(8 Pt A):2432–8.

13. Chin KJ, El-Boghdadly K. Mechanisms of action of the erector spinae plane (ESP) block: a narrative review. Can J Anaesth. 2021; 68:387–408.

Fig. 1.
Ultrasonographic visualization of a costotransverse foramen block (case 1). Color Doppler was used to confirm local anesthetic administration. ESM: erector spinae muscle, NR: neck of the rib, TP: transverse process. Yellow arrow: needle pathway.
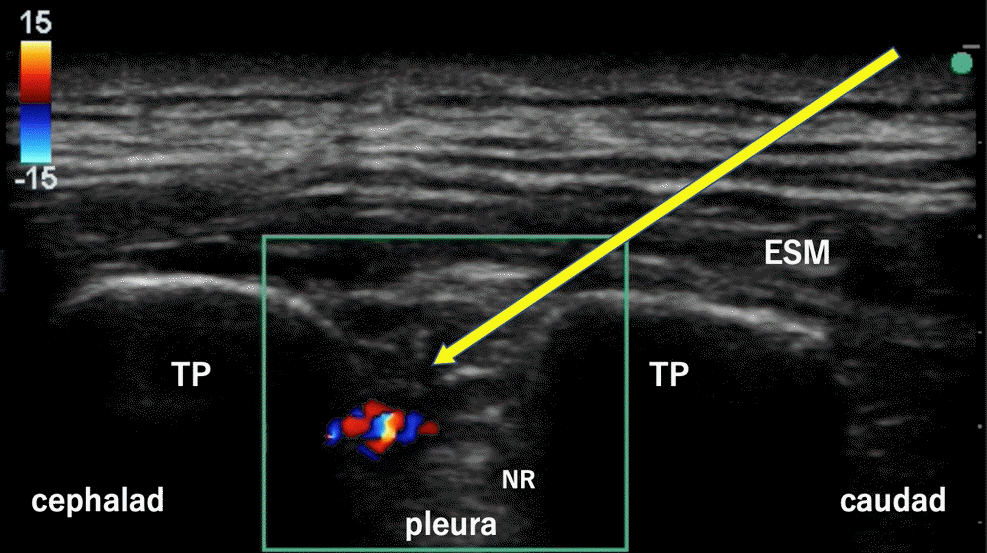
Fig. 2.
Ultrasound image after catheterization into the erector spinae plane (case 1). Normal saline solution was used to confirm the correct catheter insertion position. ESM: erector spinae muscle, NS: normal saline solution, TP: transverse process. White arrowhead: catheter.
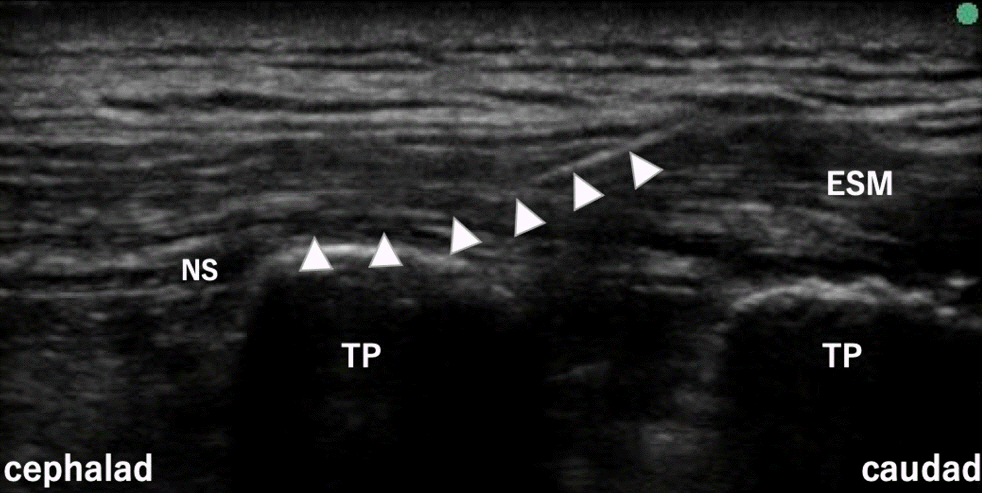
Fig. 3.
Schema representing the point of local anesthetic administration by the nerve blocks. ESPB targets the erector spinae plane, PVB targets deeper than the SCTL, and CTFB and MTPB target sites deeper than the erector spinae plane but shallower than the SCTL. These blocks allow the local anesthetic to reach the PVS more easily compared to ESPB (orange arrows). It should be noted that the puncture point and CTFB image can only be obtained by moving the probe slightly more medially. CTFB: costotransverse foramen block, ESPB: erector spinae plane block, MTPB: mid-point transverse process-to-pleura block, PVB: paravertebral block, PVS: paravertebral space, SCTL: superior costotransverse ligament, TP: transverse process.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download