Abstract
Background
Pleurisy is an inflammation of the parietal pleura and is characterized by pleuritic pain. The most common cause of pleurisy is infection; other causes include rheumatoid arthritis, malignancy, rib fractures, or trauma. Possible causes of chest pain associated with golf include costochondritis, stress fractures of the ribs, intercostal muscle strain, or, rarely, Tietze’s syndrome and slipping rib syndrome.
Case
A 64-year-old female presented with intractable chest pain that began 4 months prior while playing golf. No specific cause was found after various examinations. There was persistent pain despite medical treatment. Ultrasonography (US) was performed over the painful areas, which revealed focal pleural effusions. A mixture of ropivacaine and triamcinolone was injected into the focal pleural effusions using US guidance, which dramatically relieved her pain.
When a patient presents with intractable chest pain, physicians commonly consider potentially life-threatening disorders, such as pulmonary embolism, myocardial infarction, aortic dissection, pericardial effusion, pneumothorax, and malignancy [1]. Pneumonia, rheumatoid arthritis, tuberculosis, rib fractures, and trauma can be other causes of pain [2]. A complete medical history, physical examination, serologic studies including cardiac enzymes, electrocardiography (EKG), simple chest radiologic studies, echocardiography, computed tomography (CT), magnetic resonance imaging, and bone scans are useful for the differential diagnosis of intractable chest pain [3]. In addition, ultrasonography (US) is also a useful diagnostic tool [4]. Herein, we present a case of unusual intractable chest pain caused by playing golf, diagnosed and treated using US, in which the underlying pathology had not been found over 4 months despite various diagnostic tests.
The patient in this case was informed regarding the study and written consent was obtained for the publication of this case report.
A 64-year-old woman presented with intractable left anterior chest pain. Two years prior to the consultation, transforaminal interbody fusion and posterior spinal fusion at L5-S1 had been performed. Pain developed 4 months prior, while playing golf, characterized as a sudden stabbing type of pain; it persisted and worsened even with small movements of the arm. The intensity of pain was rated as 7–10/10 on the numerical rating scale (NRS). At that time, she consulted a private orthopedic surgeon and underwent radiological examination, which revealed no significant findings. She was administered trigger point injections and intercostal nerve blocks, which did not improve the pain. The patient was referred to a tertiary general hospital for further evaluation, as the pain persisted.
Two months earlier, she had consulted a thoracic surgeon, cardiologist, pulmonologist, and gastroenterologist. The patient underwent various examinations, such as cardiac enzyme level determination, EKG, echocardiography, coronary angiography, simple radiologic studies of the chest and bony thorax, lung high-resolution computed tomography (HRCT), esophagogastroduodenoscopy, and bone scan; however, there were no significant findings (Fig. 1).
The patient had difficulties and severe limitations in her daily life activities due to continuous and intractable pain; thus, she was transferred to our pain clinic for further evaluation and management. Physical examination revealed two painful areas on the left anterior chest (T3, T5), which were non-tender and there was no redness or swelling that indicated an infection. The pain was aggravated by flexion and extension, but there was no increased intensity or relief associated with change in position and respiration. On US examination, a linear transducer (4–18 MHz, eL18-4 Linear Probe, EPIQ 5, Philips, USA) was placed over the two painful areas, and a small pleural effusion was found (Fig. 2).
The US probe was placed transversely on the areas in which the patient felt the pain. After confirming focal pleural effusion, a 23-gauge 5 cm needle was inserted using the in-plane technique from the lateral to the medial side of the left anterior chest at a point approximately 1 cm from the probe. US-guided intralesional injection at the left T3 and T5 anterior chest was administered. A mixture of 0.75% ropivacaine hydrochloride (5.5 ml), triamcinolone acetonide (20 mg), and hyaluronidase (1,500 IU), total 6 mL was injected (Fig. 3). Pain was reduced from 8–9/10 to 0/10 based on NRS immediately after injection, and no pain was reported for approximately 1 week on telephone follow-up. Naproxen (1,000 mg/D) was prescribed to control pain and inflammation.
At the one-month follow-up, the pain was found to have reduced from 7/10 to 1–2/10 based on the NRS. An US-guided intralesional injection at the left T3 and T5 anterior chest was re-administered per the patient’s request. Two months after the first dose of intralesional injections, the patient was pain-free and had no impairment in daily life activities; thus, further follow-up was advised only if pain recurred.
Pleurisy, also called pleuritis, is an inflammation of the parietal pleura [1]. Pleuritic chest pain is characterized by sudden and intense sharp, stabbing, or burning pain in the chest and is exacerbated by forceful movements such as deep breathing and coughing [3]. The parietal pleura of the outer rib cage and lateral aspect of each hemidiaphragm are innervated by the intercostal nerves. Trauma or inflammation in these regions results in pain localized to the cutaneous distribution of these nerves [3].
Pleuritic chest pain can be caused by several factors. Life-threatening disorders, such as pulmonary embolism, myocardial infarction, aortic dissection, pericardial effusion, and pneumothorax, must first be identified [1]. Viral or bacterial pneumonia, rheumatoid arthritis, malignancy, tuberculosis, rib fractures, and trauma can be other causes [2,5]. If a cardiac or vascular source is considered, EKG, cardiac enzyme studies, and echocardiography are required [3]. When infection is considered, a complete blood count, serology, and cultures of the blood or sputum may be indicated [3].
If pleurisy is suspected, a chest X-ray examination may be performed first. This is because a chest X-ray examination, which is the least expensive and time-consuming examination available, makes it simple to screen for additional causes like pneumonia or fractures [6]. In the postero-anterior view, pleural effusion is detectable when the volume of accumulated fluid is more than 200 ml, and the lateral decubitus view can be used to check the free flow of an effusion of more than 50 ml around the lung [6]. Chest CT shows pleurisy, which is not visible in chest X-ray examinations and helps to identify the causes of effusions such as pneumonia, cancer, and pulmonary embolism [6]. However, it is difficult to distinguish small effusions from pleural thickening, dependent atelectasis, or tumors [6]. US is a useful tool for physicians managing pleural diseases, and it is particularly sensitive because of its superficial location on the body [4]. The advantages of US are rapidity, ease of use, repeatability, and no radiation exposure [6].
The higher the transducer frequency, the better the resolution, but the lower the penetration; thus, the 7.5–10 MHz probe is employed to view the pleura, and the 3.5–5 MHz probe is used to observe structures deeper in the thorax or adjacent abdominal structures in addition to the normal lung surface [7]. US images of the chest wall show soft-tissue echogenicity with multiple layers of muscle and fascia [7]. Beneath the chest wall, the parietal pleura lining the bony thorax and the visceral pleura covering the lungs are seen as two thin, bright echogenic lines, and normally, they are smooth and less than 2 mm in thickness [7].
Pleurisy is well visualized on pleural US. The parietal pleura is thickened and hypoechoic and stranding within pleural effusion may develop [5]. Pleural US identifies undulating, threadlike bands that float freely in the pleural effusion [5]. US is suitable for the identification of fluid collections throughout the body because fluid is relatively echo-free between the visceral and parietal pleura, compared with other body tissues; thus, the sonographer may readily identify even small-sized pleural effusions [7]. Pleural US can even detect small physiologic amounts of pleural fluid (less than 5 ml) and detects septations within the pleural fluid with greater sensitivity than CT scanning [6]. Furthermore, US is helpful to identify effusions based on sonographic characteristics, which include anechoic, septation, homogenously echogenic, or complex [4]. Pleural thickening, pleural tumors, pleuritis, and pneumothorax can also be detected easily and accurately with chest US [4]. Moreover, US guidance improves the rate of successful pleural aspiration [4]. Therefore, chest US can supplement other imaging modalities for the chest and guide a variety of diagnostic and therapeutic procedures [7]. The pitfall of lung US is that image artifacts are common. Bone shadowing and lung air reflection artifacts are predictable problems [7]. The probe should be moved in transverse or longitudinal directions along the intercostal spaces to avoid interference by the bony ribs [7].
In this case, the patient underwent various examinations, such as echocardiography and coronary angiography, HRCT of the lung, and bone scan, which showed no specific findings. However, focal pleural effusion was found using US of the localized painful areas; therefore, pleural US was the key for evaluation. In this case, none of the above test results are specific, and if a patient complains of persistent localized chest pain, performing US on the painful area may be helpful in the diagnosis and treatment.
Considering that the golf swing is an action that uses the maximum possible rotational force, several causes of chest pain can be associated with it. First, injuries to the chest from continuous, repetitive rotational movements can cause costochondritis, which is an inflammation of the cartilage that connects the ribs to the sternum [8]. Second, athletes may also experience Tietze’s syndrome, which is an inflammatory disorder that affects the chest wall cartilage [9]. Third, stress fractures of the ribs are common and can be diagnosed using a bone scan [10]. Fourth, slipping rib syndrome, which is an intercostal nerve impingement resulting from abnormal movement of false ribs related to unstable costal cartilage attachments, can be caused by the maximum possible rotation of the thoracic spine [11]. Fifth, intercostal muscle strain or pull can occur when the muscle is stretched or torn due to excessive rotation, which may also cause sharp chest pain.
In this case, we postulated three hypotheses that could have caused focal pleural effusion that led to intractable chest pain. First, as the pain began while playing golf, we assumed that the microfracture on the inferior side of the rib adjacent to the parietal pleura may have occurred four months prior to the consultation. It may have continued to stimulate the pleura and caused inflammation, a small effusion, and chronic chest pain. However, the bone scan that was performed did not reveal significant findings. The second possible theory is interposition of the costal cartilage. Considering the swing-rotation posture used by golfers, the rib-costal cartilage and costal cartilage-costal cartilage would overlap each other, and focal pleural inflammation may be caused by pinching or friction of the pleura. Third, because of excessive rotation, costochondral inflammation due to costosternal joint deformity may have resulted in a costochondral degenerative change and progressed to costochondral osteoarthritis. Persistent costochondral inflammation may have led to pleural irritation, resulting in focal pleural effusion.
After excluding life-threatening causes of pleuritic chest pain that require emergent treatment, pain control and treatment of the etiology of underlying conditions should be performed [3]. Nonsteroidal anti-inflammatory drugs are commonly prescribed as the initial therapy for pain control, since side effects with these are significantly less frequent than those with opioids, which are centrally acting analgesics [1]. Corticosteroids inhibit many of the initial events in an inflammatory response and promote the resolution of inflammation by inhibiting vasodilation, so that leukocyte emigration into inflamed sites is decreased [12]. In addition, there are case reports in which inflammation and pain improved after intrapleural corticosteroid injection in eosinophilic pleural effusion and tuberculous pleural effusion [13,14]. In this case, the cause of focal pleural effusion, which caused persistent pain in the patient, was probably inflammation in that area, and the patient wanted improvement in intractable chest pain urgently. An intralesional injection was administered by US for diagnostic and therapeutic purposes. In this process, a small amount of colorless fluid was aspirated, but due to the risk of pneumothorax, we could not obtain enough fluid for pleural fluid examination. The regimen selected for administration by injection was a local anesthetic for pain control and a steroid for inflammation control.
In conclusion, this case demonstrates that US should be considered as a diagnostic modality for unusual chest pain that has not been diagnosed despite various examinations, since pleurisy could be well visualized by pleural US. US-guided intralesional injection can be a treatment option for patients with focal pleural effusions.
Notes
REFERENCES
1. Kass SM, Williams PM, Reamy BV. Pleurisy. Am Fam Physician. 2007; 75:1357–64.
2. Hunter MP, Regunath H. Pleurisy. StatPearls. In : Abai B, Abu-Ghosh A, Acharya AB, Acharya U, Adhia SG, Sedeh PA, editors. Treasure Island (FL): StatPearls Publishing;2021.
3. Reamy BV, Williams PM, Odom MR. Pleuritic chest pain: sorting through the differential diagnosis. Am Fam Physician. 2017; 96:306–12.
5. Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA. 1997; 278:1440–5.

6. Hooper C, Lee YC, Maskell N; BTS Pleural Guideline Group. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax. 2010; 65 Suppl 2:ii4–17.

7. Tsai TH, Yang PC. Ultrasound in the diagnosis and management of pleural disease. Curr Opin Pulm Med. 2003; 9:282–90.

8. Gregory PL, Biswas AC, Batt ME. Musculoskeletal problems of the chest wall in athletes. Sports Med. 2002; 32:235–50.

10. Miller TL, Harris JD, Kaeding CC. Stress fractures of the ribs and upper extremities: causation, evaluation, and management. Sports Med. 2013; 43:665–74.

11. Choi JB, Yoon KB, Kim WO, Yoon DM. Treatment of a twelfth rib syndrome: a case report. Korean J Pain. 2009; 22:96–8.

12. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011; 335:2–13.

Fig. 1.
(A) High-resolution computed tomography image of the chest, which was initially read as normal, but upon reexamination, was found to show focal pleural effusion (white arrow), (B) Magnified view of the left anterior chest showing focal pleural effusion (white arrow).
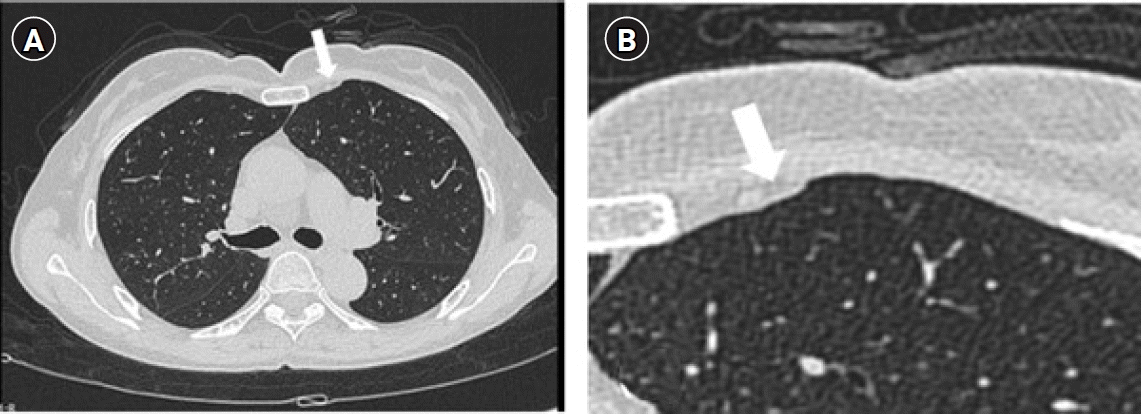
Fig. 2.
An ultrasonographic image of the focal pleural effusion asterisk (*). A long axis view of the anterior intercostal space at T5–6 with 4–18 MHz linear transducer (EPIQ 5, Philips, USA).
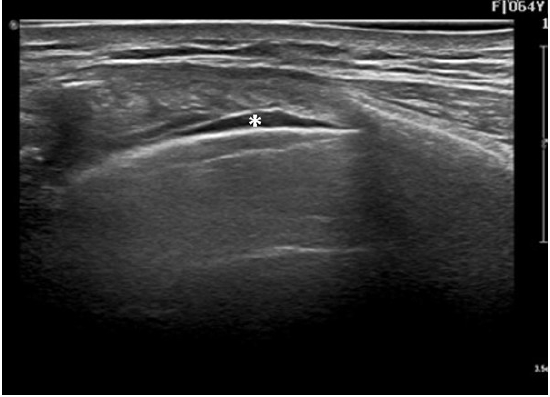
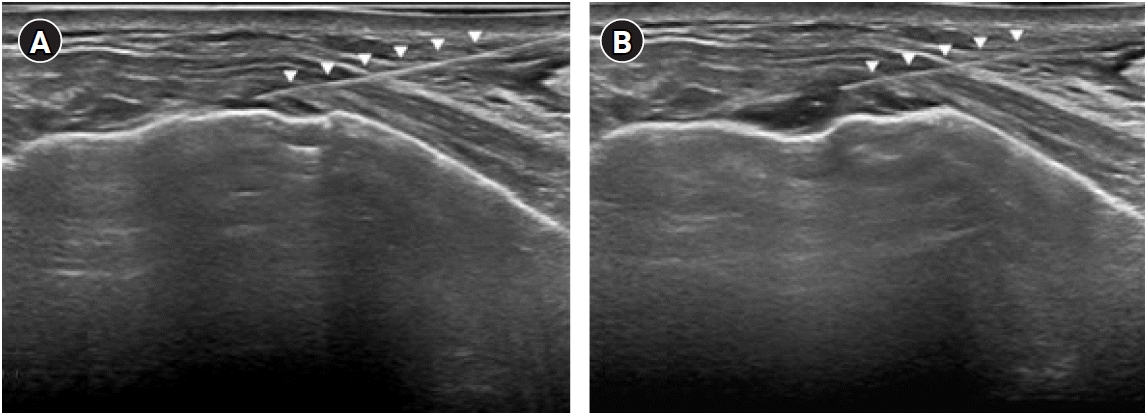
Fig. 3.
An image of the ultrasound-guided intralesional injection. The white arrowhead indicates the needle. A long axis view of the anterior intercostal space at T5–6 with a 4–18 MHz linear transducer (EPIQ 5, Philips, USA). (A) Confirming the focal pleural effusion, a 23-gauge 5 cm needle was inserted using the in-plane technique from the lateral to the medial side of the left anterior chest at a point about 1 cm from the probe. (B) Intralesional injection was administered.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download