INTRODUCTION
MATERIALS AND METHODS
Patients and data collection
BNP measurement
RCRI and post-reperfusion syndrome (PRS)
Outcomes
Statistics
RESULTS
Table 1.
| Variable | DeltaBNP > 0 (n = 3,217) | DeltaBNP < 0 (n = 594) | Total (n=3,811) | P value |
|---|---|---|---|---|
| Age (yr) | 54.0 (49.0, 59.0) | 54.0 (47.0, 60.0) | 54.0 (48.0, 59.0) | 0.139 |
| Male | 2,368 (73.6) | 401 (67.5) | 2,769 (72.7) | 0.003* |
| MELD score | 14 (9, 23) | 26 (14, 37) | 15 (10, 26) | < 0.001* |
| Viral liver disease | 2,050 (63.7) | 269 (45.3) | 2,319 (60.9) | < 0.001* |
| Alcoholic liver disease | 655 (20.4) | 178 (30.0) | 833 (21.9) | < 0.001* |
| Diabetes | 810 (25.2) | 133 (22.4) | 943 (24.7) | 0.163 |
| Hypertension | 592 (18.4) | 120 (20.2) | 712 (18.7) | 0.329 |
| RCRI | < 0.001* | |||
| 1 | 2,225 (69.2) | 299 (50.3) | 2,524 (66.2) | |
| 2 | 789 (24.5) | 220 (37.0) | 1,009 (26.5) | |
| 3 | 203 (6.3) | 75 (12.6) | 278 (7.3) | |
| Intractable ascites | 991 (30.8) | 257 (43.3) | 1,248 (32.7) | < 0.001* |
| Orthotopic LT | 446 (13.9) | 231 (38.9) | 677 (17.8) | < 0.001* |
| Hydrothorax | 438 (13.6) | 99 (16.7) | 537 (14.1) | 0.057* |
| Creatinine (mg/dl) | 0.8 (0.6, 1.0) | 1.0 (0.7, 1.8) | 0.8 (0.6, 1.1) | < 0.001* |
| INR | 1.4 (1.2, 1.8) | 1.7 (1.4, 2.3) | 1.4 (1.2, 1.9) | < 0.001* |
| Total bilirubin (mg/dl) | 2.0 (1.0, 6.9) | 9.2 (1.9, 25.1) | 2.2 (1.0, 10.5) | < 0.001* |
| pRBC (units) | 8.0 (3.0, 16.0) | 12.0 (6.0, 20.5) | 8.0 (3.0, 16.0) | < 0.001* |
| PRS | 1,940 (60.3) | 377 (63.5) | 2,317 (60.8) | 0.160 |
| BNP (pg/ml) | 43 (21, 92) | 251 (118, 586) | 54 (24,130) | < 0.001* |
| postBNPPOD3 (pg/ml) | 197 (95, 423) | 132 (73, 287) | 183 (90, 405) | < 0.001* |
| DeltaBNP (pg/ml) | 130 (52, 330) | -76 (-241, -24) | 95 (24, 273) | < 0.001* |
| MACE | 493 (15.3) | 144 (24.2) | 637 (16.7) | < 0.001* |
| 30-day mortality | 64 (2.0) | 36 (6.1) | 100 (2.6) | < 0.001* |
Values are presented as median (1Q, 3Q) or number (%). BNP: B-type natriuretic peptide, MELD: model for end-stage liver disease, RCRI: revised cardiac risk index, LT: liver transplantation, INR: international normalized ratio, pRBC: packed red blood cells transfused, PRS: post-reperfusion syndrome, postBNPPOD3: peak BNP levels within the first 3 postoperative days, MACE: major adverse cardiovascular events.
Characteristics in patients with deltaBNP < 0
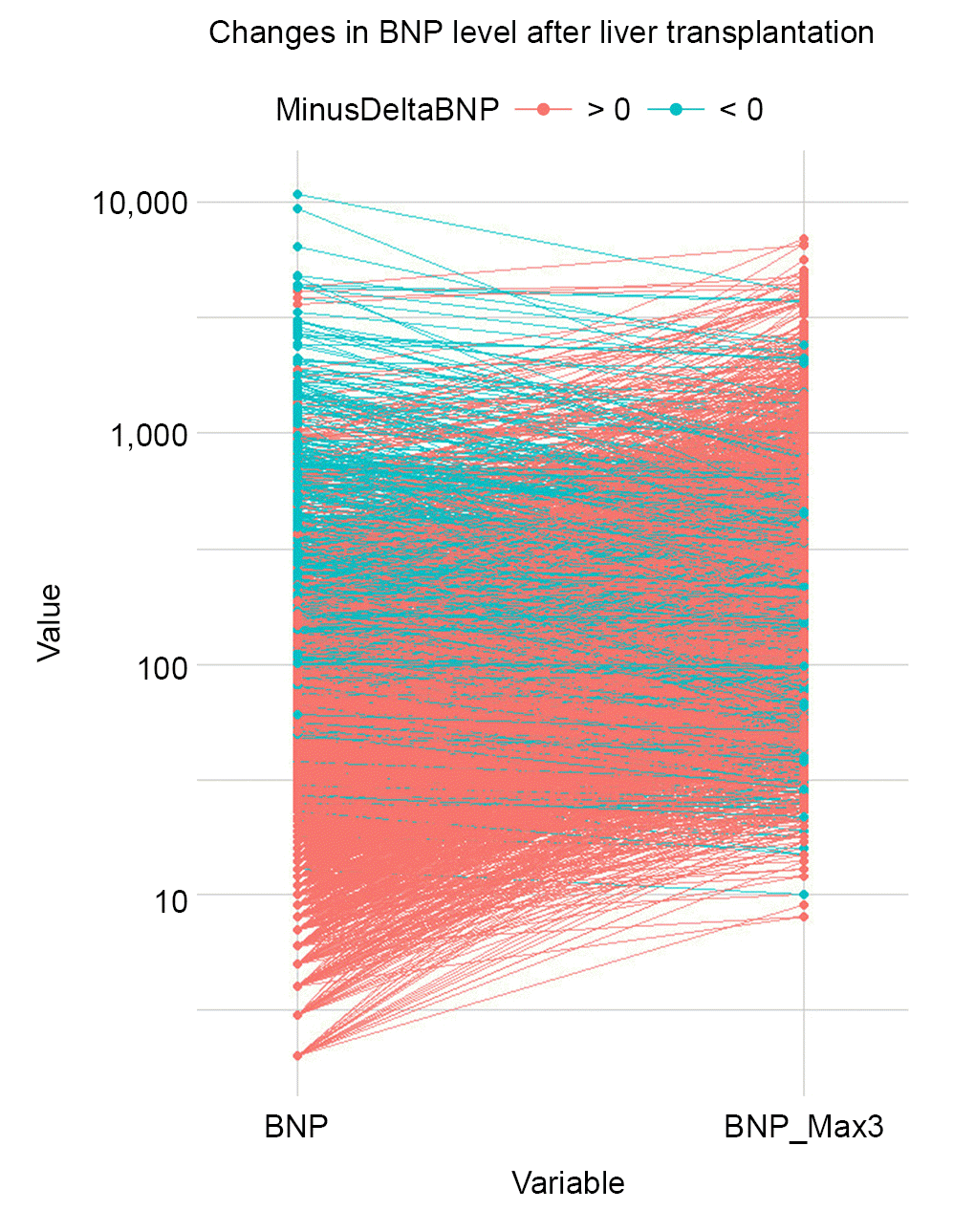 | Fig. 1.Line plots depicting individual value preoperative B-type natriuretic peptide (BNP) and BNP_Max3 liver transplantation. Line shows the change of preoperative BNP value to the BNP_Max3. BNP: B-type natriuretic peptide, BNP_Max3: high BNP levels within the first 3 postoperative days, DeltaBNP: difference between BNP_Max3 and preoperative BNP. |
Thirty-day mortality and MACE
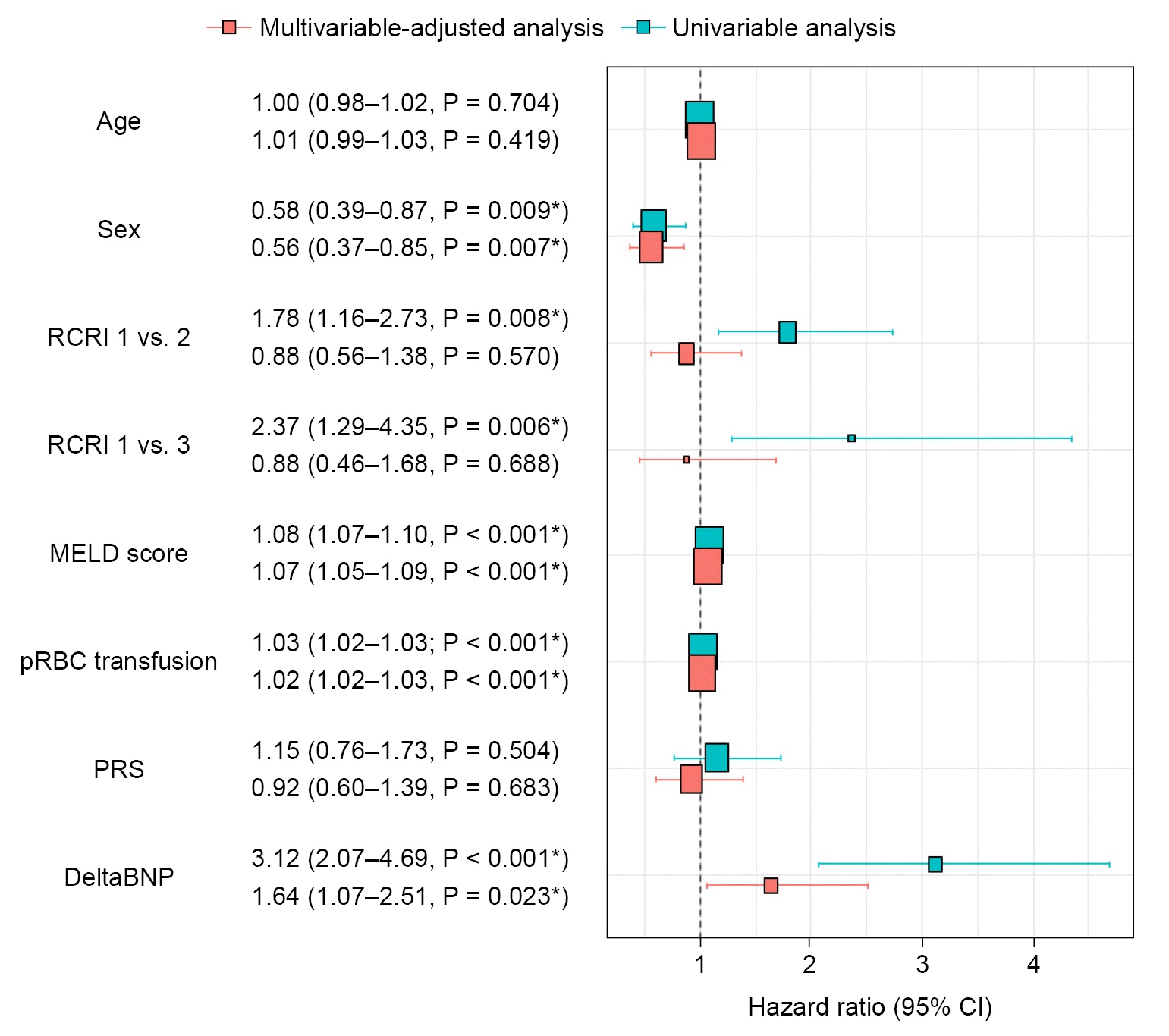 | Fig. 3.Hazard ratios of univariable and multivariable analysis for 30-day mortality. RCRI: revised cardiac risk index, MELD: model for end-stage liver disease, pRBC: packed red blood cells transfused, PRS: post-reperfusion syndrome, BNP: B-type natriuretic peptide, CI: confidence interval. *P < 0.05 was considered statistically significant. |
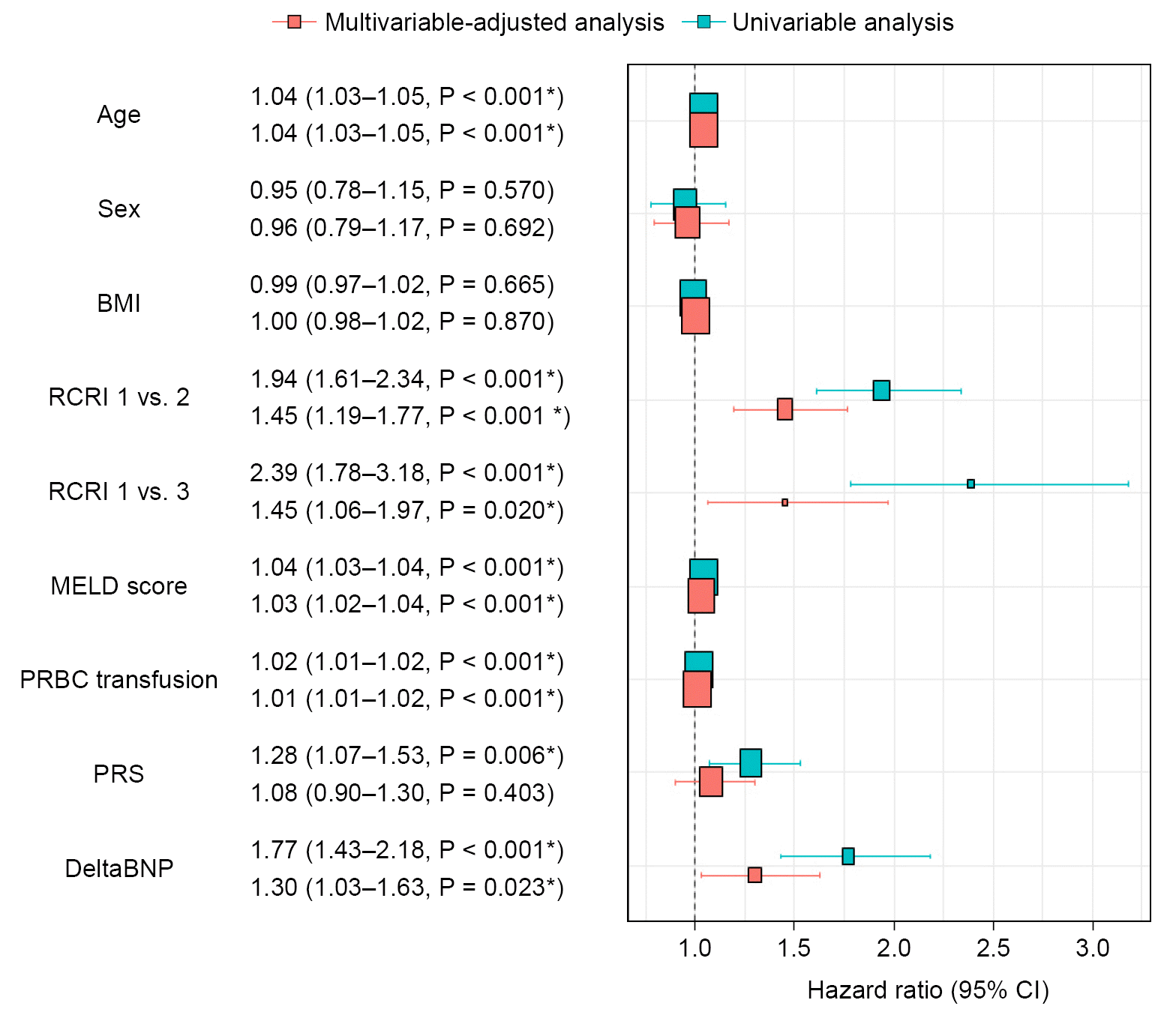 | Fig. 4.Odds ratios of univariable and multivariable analysis for major adverse cardiovascular events (MACE). BMI: body mass index, RCRI: revised cardiac risk index, MELD: model for end-stage liver disease, pRBC: packed red blood cells transfused, PRS: post-reperfusion syndrome, BNP: B-type natriuretic peptide, CI: confidence interval. *P < 0.05 was considered statistically significant. |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download