This article has been
cited by other articles in ScienceCentral.
Abstract
High-intensity focused ultrasound (HIFU) ablation is a safe and effective minimally invasive option for treating uterine fibromas, but it can cause complications such as abdominal wall defects. Reconstruction of such defects can be challenging, but a latissimus dorsi musculocutaneous free flap can be used to restore the integrity of the myofascial layer and provide external cutaneous coverage. Herein, we present a case report of a 37-year-old woman who underwent HIFU for an 8-cm uterine fibroma and subsequently developed a large abdominal wall defect with necrosis of the rectus muscle. The latissimus dorsi musculocutaneous free flap was used to reconstruct the rectus muscle and cover the abdominal soft tissue, resulting in successful engraftment without complications. We present our experience using a latissimus dorsi musculocutaneous free flap to reconstruct a large HIFU-induced composite defect in the abdominal wall.
Go to :

Keywords: Abdominal wall defect, Latissimus dorsi free flap, Musculocutaneous flap
Introduction
High-intensity focused ultrasound (HIFU) has a role in ablating tumors by changing the frequency, which is unlike that of diagnostic ultrasound. In the treatment of uterine fibromas, HIFU ablation is considered to be one of the minimally invasive options for patients who preserve the uterus [
1,
2]. Although it is safe and effective, the HIFU ablation is known to cause mild complications such as skin burn, vaginal discharge, and abdominal pain to severe complications such as abdominal wall defect, intestinal perforation, and uterine bleeding [
2]. Among them, abdominal wall defect is very challenging for reconstruction surgeons, if the defect is too large and deep to be covered with direct closure or local flap. In particular, when the abdominal defect involves the rectus abdominis muscle, it can be a serious problem. This full-thickness loss of the abdominal wall musculofascial and overlying soft tissue—a composite defect—require not only external cutaneous coverage but also muscle repair [
3,
4]. We present our experience using a latissimus dorsi musculocutaneous free flap for the reconstruction of HIFU-induced abdominal wall large composite defect.
Go to :

Case report
This study was approved by the Institutional Review Board of Hanyang University Medical Center (IRB No. 2023-05-057). Written informed consent was obtained from the patient for the publication of this report including all clinical images.
A 37-year-old unmarried woman with no underlying diseases underwent HIFU for the treatment of 8-cm uterine fibroma. Abdominal pain persisted one month after the procedure, and the patient was hospitalized and received conservative therapy such as IV antibiotics, but the abdominal pain did not improve. Afterward, the pain worsened and urinary retention developed, so a computed tomography (CT) scan was performed, and findings of bladder rupture and rectus abdominis muscle necrosis were observed. Eventually, the patient underwent an emergency laparotomy, and the urology team successfully performed the repair of the bladder rupture.
However, the main problem is the contamination of the abdominal cavity with urine due to bladder rupture and consequent necrosis of the rectus muscle about two-thirds. As a result, our plastic surgery team and gynecology team cooperatively performed debridement on the wound bed and unviable tissues, which resulted in about 20×15-cm sized abdominal wall fascia defect and 15×5-cm sized soft tissue defect. Due to shrinkage, the defect size of the rectus muscle and peritoneum could not be measured (
Fig. 1A). Although there was a history of urine contamination, the wound bed did not present an infectious condition such as an abscess. After removing nonviable tissue, the defect site was relatively clean and suitable for immediate reconstruction. For reconstruction of the rectus muscle and coverage of abdominal soft tissue, we decided to perform a latissimus dorsi musculocutaneous free flap. Right deep inferior epigastric artery had suffered thermal damage of previous HIFU, and no internal blood flow was observed, so it was ligated to prevent unexpected bleeding. Fortunately, the left deep inferior epigastric artery was relatively patent and suitable as the recipient’s vessel. While maintaining the supine position without position change, we harvested the latissimus dorsi musculocutaneous free flap from the right axillary area. Due to the large size of the rectus muscle defect, we harvested approximately 20×15 cm of latissimus dorsi muscle flap, and about 15×5 cm of skin flap was harvested considering the need for a monitoring flap. The pedicle length of the flap was 10 cm (
Fig. 1B). Myofascial defect was repaired by latissimus dorsi muscle flap with anastomosis to left deep inferior epigastric vessels (1 artery and 1 vein fashion, end-to-end anastomosis, each vessel). The harvested latissimus dorsi muscle was incorporated into the abdominal wall defect and sutured to the remaining rectus muscle and sheath. The latissimus dorsi muscle fascia was tied to the Scarpa’s fascia in the abdomen to prevent abdominal hernias. No other materials, such as artificial mesh or alloplastic dermis, were used to reinforce the abdominal wall. The skin flap was de-epithelized to cover the soft tissue defect and the remaining skin flap was used to be a monitoring flap (
Fig. 1C).
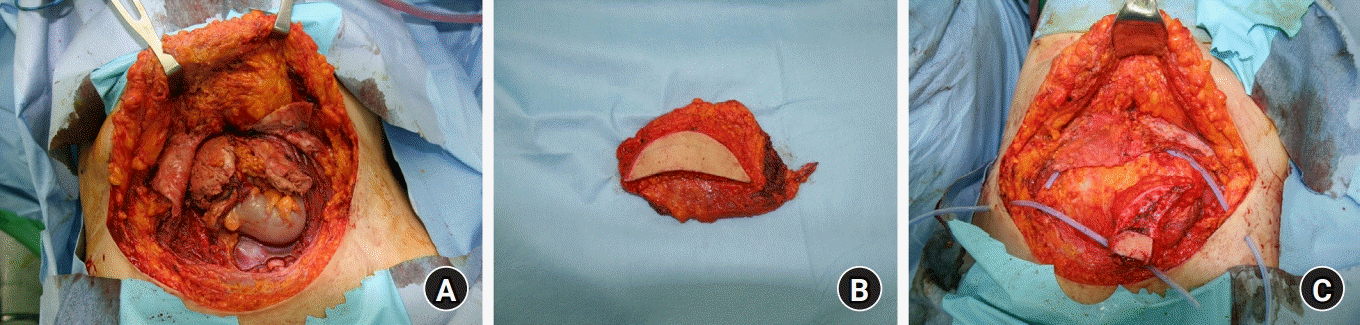 | Fig. 1.(A) After debridement of necrotic and unviable tissue, the abdominal wall fascia defect measured about 20×15 cm and the soft tissue defect measured roughly 15×5 cm with loss of the rectus abdominis muscle about 2/3 from the origin. (B) An approximately 20×15-cm latissimus dorsi musculocutaneous flap was harvested with a 10 cm-long pedicle in the right axillary area. (C) The myofascial defect was repaired by latissimus dorsi muscle and fascia with anastomosis to the left deep inferior epigastric vessels. A 15×5-cm skin flap was de-epithelized to cover the soft tissue defect, and the remaining skin flap was used to be monitoring flap. 
|
In the follow-up CT scan 2 weeks later, because the muscle layer of the flap could not be used immediately after surgery, a part of the flap was fatty degeneration [
5] (
Fig. 2). But it can be seen that the flap was engrafted well overall. Also, soft tissue defect was all covered without complications such as infection, seroma, and herniation. Although a minor hypertrophic scar did develop, it was easily corrected through scar revision while removing the monitoring flap (
Fig. 3).
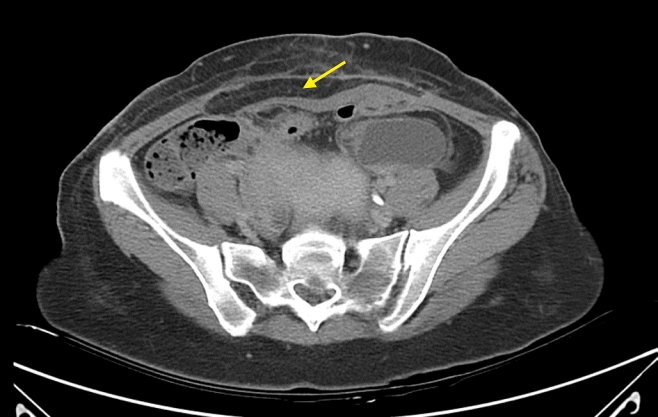 | Fig. 2.A follow-up computed tomography scan taken 2 weeks later. Although part of the flap exhibited fatty degeneration (yellow arrow), the latissimus dorsi musculocutaneous free flap was inset well overall in the defect area in the abdomen. 
|
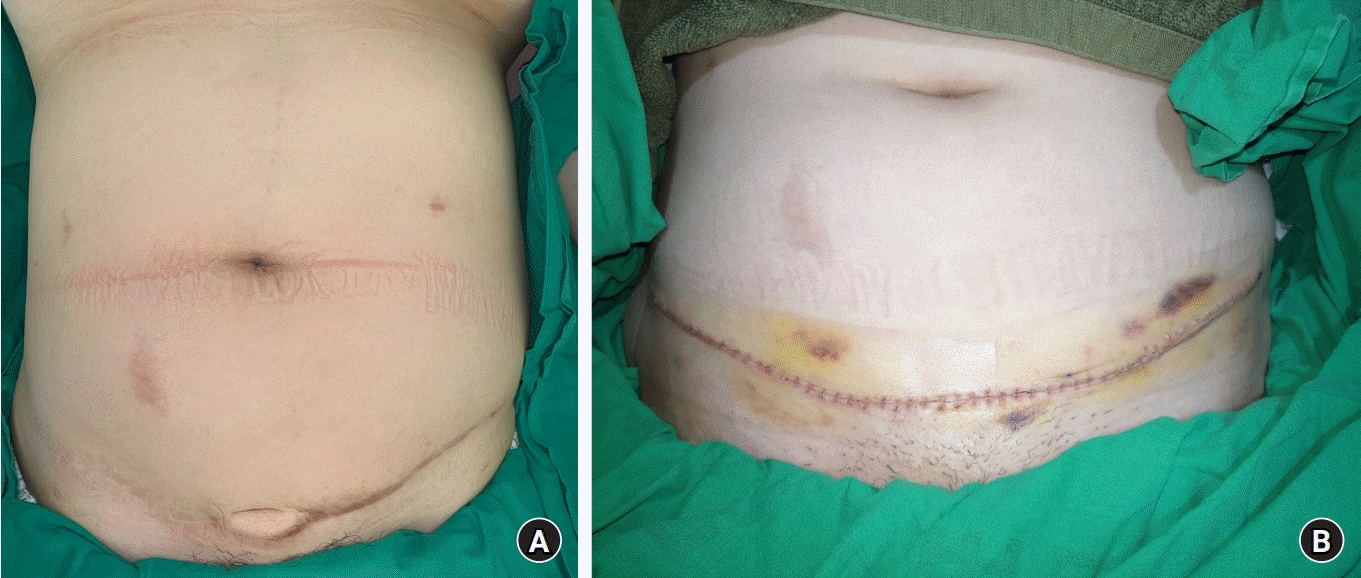 | Fig. 3.(A) Long-term follow-up view showed good coverage, with a mild hypertrophic scar. (B) One year after the free flap operation, scar revision was done with detachment of the monitoring flap. 
|
Go to :

Discussion
The reconstruction of large full-thickness abdominal wall defects is challenging for reconstruction surgeons. The goals of abdominal wall reconstruction are to re-establish the integrity of the myofascial layer and provide external cutaneous coverage [
3,
6]. In our case, the patient's abdominal wall defect was particularly challenging due to the large size of the composite defect involving the rectus abdominis muscle. We opted to use a latissimus dorsi musculocutaneous free flap for reconstruction, which allowed us to repair the huge myofascial defect and cover the soft tissue defect with a reliable donor site. The latissimus dorsi flap can offer various functional units including skin, soft tissue, muscle, fascia, and bone. The advantage and versatility of thoracodorsal axis flap have been well documented in previous reports, especially in the reconstruction of extensive orofacial defects and complicated defects of the lower extremities. These properties impart superior application in the reconstruction of extensive, complex, or three-dimensional defects [
7,
8].
When reconstructing composite full-thickness defects like our case including skin, soft tissue, fascia, and muscle, it is necessary to obliterate the dead space while resurfacing the defect to prevent complications such as wound infection and abscess formation, especially when contaminated [
3]. Because the principle was applied using latissimus dorsi musculocutaneous flap, complications such as infection, abscess could be prevented in our case.
Although there was some fatty degeneration of the flap in the CT scan, we believe this was due to the restriction of rectus muscle movement for about 1 to 2 weeks to ensure free flap stability. Furthermore, even though we performed muscle repair without neurorrhaphy, we think that such non-functional muscle transfer carries several implications on its own. Firstly, in reconstructing full-thickness large abdominal wall defects like this case, we believe that not only functional reconstruction but also aesthetic aspects should be considered. The latissimus dorsi muscle serves as a structure to fill the depression of the full-thickness defect three-dimensionally. Secondly, there was intraperitoneal contamination due to a bladder rupture. In such condition, using artificial mesh or alloplastic dermis for abdominal wall reinforcement at the defect site to prevent hernia would likely increase the risk of postoperative infection. Instead, we used the fascia of the latissimus dorsi muscle to tie it to the remaining Scarpa’s fascia to reinforce abdominal wall. Thirdly, by insetting a well-vascularized and sufficient amount of latissimus dorsi muscle into the infected defect site, we believe that wound healing can be enhanced and the local infection or spreading of bacterial inoculation can be prevented [
8]. At 6 months of follow-up observation, the patient reported no significant discomfort in exercising the rectus muscle and abdominal herniation. As can be seen from the CT scan, the overall engraftment was successful, and the patient’s soft tissue defect was fully covered without major complications.
We believe that the latissimus dorsi musculocutaneous free flap is a good option for the reconstruction of large full-thickness abdominal wall defects, particularly when the defect involves the rectus abdominis muscle such as in our case caused by HIFU ablation. While this technique requires a degree of surgical expertise, it is a reliable and effective option for patients who require abdominal wall reconstruction.
Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download