Abstract
Malignant peripheral nerve sheath tumors (MPNSTs) are rare, aggressive soft tissue sarcomas with a high rate of recurrence and metastasis. Limb salvage surgery with free flap reconstruction is a viable option for selected patients with MPNSTs, but careful consideration should be given to the risk of recurrence. This case report describes a 26-year-old male patient with a recurrent, aggressive, high-grade MPNST who underwent limb salvage surgery with thoracodorsal artery perforator free flap reconstruction. Despite the surgical intervention, local recurrence of the MPNST was detected, and below-knee amputation was ultimately recommended. This case highlights the importance of early, definitive treatment decision-making in cases of aggressive, high-grade MPNSTs. Close postoperative monitoring and early detection of recurrence are crucial for achieving optimal outcomes in patients with MPNSTs undergoing limb salvage surgery with free flap reconstruction.
Malignant peripheral nerve sheath tumors (MPNSTs) are a rare and aggressive type of soft tissue sarcomas arising from peripheral nerve or preexisting nerve sheath tumors, including neurofibroma [1]. They develop in about 8% to 13% of individuals with neurofibromatosis type 1 (NF1), a genetic disorder that causes the growth of benign and malignant tumors in the nervous system [2]. Treatment of MPNSTs typically involves surgical resection with wide margins, but the potential for local recurrence and metastasis remains a significant concern and multimodality treatment for MPNST including radiation therapy and chemotherapy is recommended [3]. In cases of MPNST recurrence, reconstruction of the resulting defect can present a challenging task for plastic and reconstructive surgeons and early definitive treatment may provide a better outcome. This case report describes the management of a recurrent MPNST on the lateral side of the right ankle in a patient with neurofibromatosis, with a focus on the use of a thoracodorsal artery perforator free flap for defect reconstruction and the challenges encountered during postoperative follow-up.
This study was approved by the Institutional Review Board of Hanyang University Medical Center (IRB No. 2023-05-056). Written informed consent was obtained from the patient for the publication of this report including all clinical images.
The patient was a 26-year-old male with a history of NF1 and MPNSTs on the medial side of the right ankle. In January 2017, he received radical resection of the tumors, and the resulting defect was covered with a thoracodorsal artery perforator free flap harvested from the left side, followed by adjuvant radiation therapy (Fig. 1A). About 69 months later, in October 2022, he presented with recurrent MPNSTs protruding on the lateral side of the right ankle involving multiple structures (Fig 1B). Preoperative ankle magnetic resonance imaging (MRI) revealed extensive, infiltrative masses involving the lateral and posterior subcutaneous fat layers of the ankle and foot, abductor digiti minimi muscle, peroneus muscle and tendon, and encasing the sural nerve, lesser saphenous vein, and Achilles tendon. Anteriorly, the masses encased the extensor digitorum brevis muscle, extensor retinaculum, and greater saphenous vein. In the case of bone involvement, bony erosions were observed in the calcaneus, talus, and distal fibula (Fig 1C).
Despite the risk of recurrence, limb salvage with wide excision of the MPNSTs and coverage with a thoracodorsal artery perforator free flap was performed. The extensive involvement of surrounding structures and infiltrative nature of recurred MPNSTs made complete surgical resection impossible. The removed mass measured 14×18 cm, and the final defect was 15×20 cm in size (Fig. 2A). To cover the defect, a 15×20 cm thoracodorsal artery perforator free flap with a 12 cm-long pedicle was harvested from the right side (Fig. 2B). The vessels of the pedicle were anastomosed to the anterior tibial artery and vena comitantes, in an end-to-side and end-to-end manner, respectively. The free flap transfer was successful, and the flap remained viable without any complications until the 8th postoperative day (Fig. 3A). On the 8th postoperative day, a distal part of the flap measuring about 1.5×4.5 cm showed a bluish color change, indicating newly occurred arterial insufficiency (Fig. 3B). The affected portion of the flap continued to extend, and by the 25th postoperative day, the final demarcated portion measured 3×12 cm in size (Fig. 3C). On the 26th postoperative day, flap revision was planned to remove the demarcated, necrotic portion of the flap, and vacuum-assisted wound closure was applied. During the revision, local recurrence and rapid growth of tumors were observed on the wound bed. Finally, 14 days after the initial vacuum-assisted wound closure, coverage with split-thickness skin graft and biopsy were performed (Fig 3D). The result of biopsy confirmed the recurrence of the tumor. Contrary to the previous treatment in 2017, adjuvant radiation therapy was not administered this time. The patient was discharged on the 45th postoperative day from the wide excision and coverage with thoracodorsal artery perforator free flap surgery.
The patient underwent regular follow-ups every week in the outpatient clinic. During the 54th postoperative day follow-up, the flap operative site exhibited significant oozing and swelling, suggesting rapid growth of recurrent MPNSTs on the wound bed. A postoperative follow-up MRI revealed rapidly growing known MPNSTs with intraarticular extension to the ankle, subtalar, and intertarsal joints, with increased involvement of the Achilles tendon (Fig. 4). At the last outpatient clinic follow-up before the patient’s referral for possible radiation therapy, the operative site showed signs of infection with the continued growth of tumors, and the ankle was deemed irreparable. As a result, amputation below the knee was recommended as a possible solution.
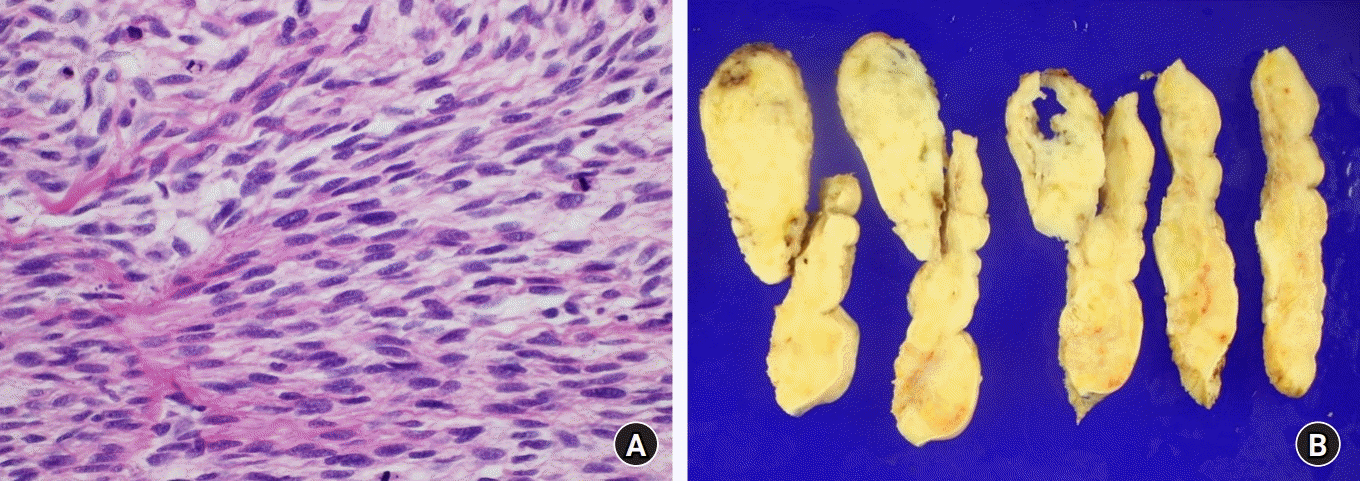
In this case report, the authors describe the challenges in managing recurrent MPNSTs which are aggressive and invasive cancers with poor prognosis, particularly in patients with NF1 [3]. Clinical suspicion of MPNST is based on factors such as a known history of NF1 and the presentation of tumors that grow rapidly in size [4]. Although the mainstay treatment of MPNSTs is surgical removal, it is always not possible due to their size and infiltrative nature [5]. Complete surgical resection is often hindered by the large size of tumors, their proximity to complex nerve networks, and the high rate of positive margins [6]. Furthermore, patients who have had incomplete excision of tumors show a significantly increased risk of local and distant recurrence, compared to those who underwent complete excision. The authors report a case in which limb salvage was prioritized due to the patient’s demand and the history of successful reconstruction, although complete resection was not feasible and the patient was at high risk of future local recurrence. Regrettably, the history of adjuvant radiation therapy on the right ankle may have increased the mutational burden of the tumor, thereby making it even more aggressive [7]. The patient’s recurred MPNST histology revealed high-grade MPNST with more than 10 mitotic figures per 10 high power field combined with necrosis (Fig. 5) [8]. On the 8th postoperative day, the stable flap showed suspicion of arterial insufficiency caused by local recurrence. On the 26th postoperative day, during the flap revision, local recurrence and regrowth of tumors were identified on the wound bed, at a rate which proceeded the authors’ prediction. Although the authors have tried to salvage young patient's limb with thoracodorsal artery perforator free flap, aggressive, infiltrative, high-grade MPNST have led to a suggestion of amputation. This case highlights the importance of making an early decision regarding definitive treatment for managing high-grade MPNSTs to improve an overall outcome of a patient.
References
1. Somatilaka BN, Sadek A, McKay RM, Le LQ. Malignant peripheral nerve sheath tumor: models, biology, and translation. Oncogene. 2022; 41:2405–21.

2. Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002; 39:311–4.

3. Stucky CC, Johnson KN, Gray RJ, et al. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. 2012; 19:878–85.

4. James AW, Shurell E, Singh A, Dry SM, Eilber FC. Malignant peripheral nerve sheath tumor. Surg Oncol Clin N Am. 2016; 25:789–802.

5. Theos A, Korf BR; American College of Physicians; American Physiological Society. Pathophysiology of neurofibromatosis type 1. Ann Intern Med. 2006; 144:842–9.

6. Farid M, Demicco EG, Garcia R, et al. Malignant peripheral nerve sheath tumors. Oncologist. 2014; 19:193–201.

7. Nakamura JL, Phong C, Pinarbasi E, et al. Dose-dependent effects of focal fractionated irradiation on secondary malignant neoplasms in Nf1 mutant mice. Cancer Res. 2011; 71:106–15.
Fig. 1.
A patient presented with malignant peripheral nerve sheath tumor (MPNST) on his right ankle. (A) In 2017, he was diagnosed with MPNST on the medial side of the right ankle and received wide excision and coverage with a thoracodorsal artery perforator free flap, followed by adjuvant radiation therapy. (B) In 2022, he presented with recurrent MPNST on the lateral side of the right ankle. (C) Sagittal view of preoperative T2-weighted magnetic resonance imaging revealed extensive involvement of surrounding structures.
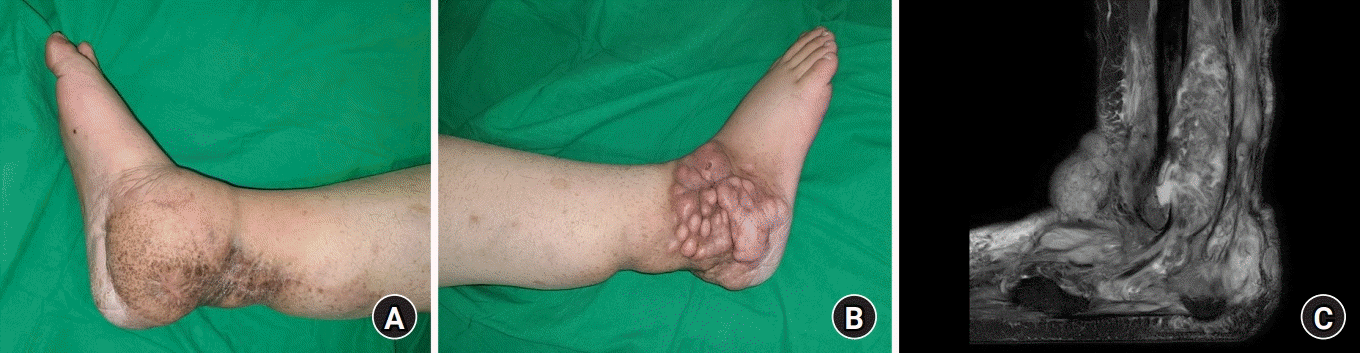
Fig. 2.
(A) Recurrent malignant peripheral nerve sheath tumor (MPNST) was removed from the lateral aspect of the right ankle with a positive margin. The main mass measured 14×18 cm. (B) A thoracodorsal artery perforator free flap was harvested from the right side for defect coverage. The flap measured 15×20 cm, with a 12 cm-long pedicle.
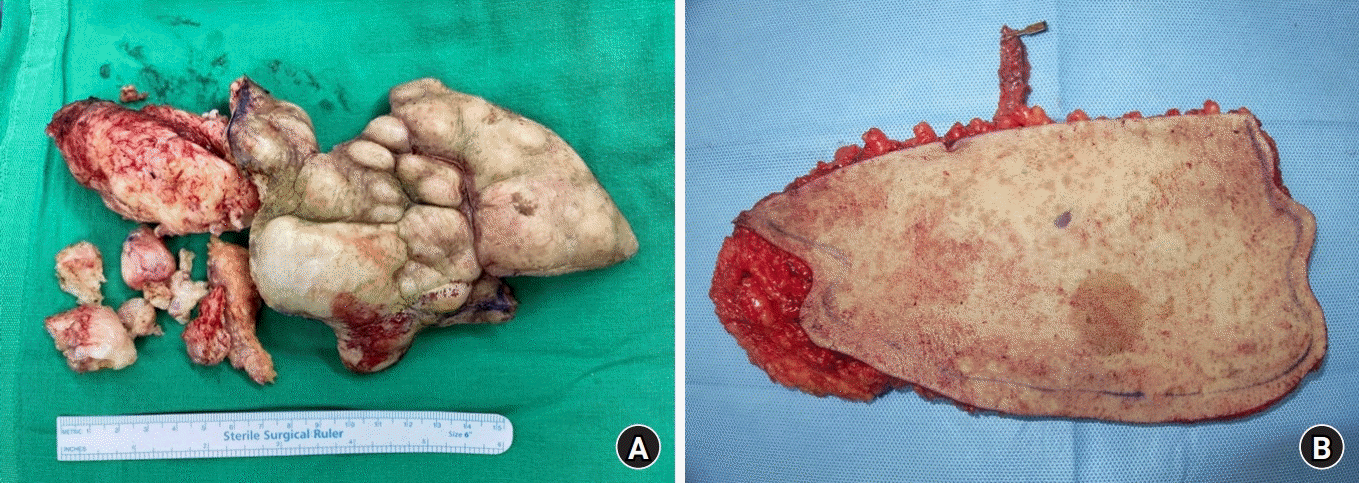
Fig. 3.
(A) Free flap transfer was performed successfully without complications. (B) On the 8th postoperative day, the distal portion of the flap showed a bluish color change suggesting arterial insufficiency. (C) By the 25th postoperative day, the final portion with arterial insufficiency measured 3×12 cm. (D) Flap revision was performed to remove the demarcated portion, and the defect was covered with a split-thickness skin graft.
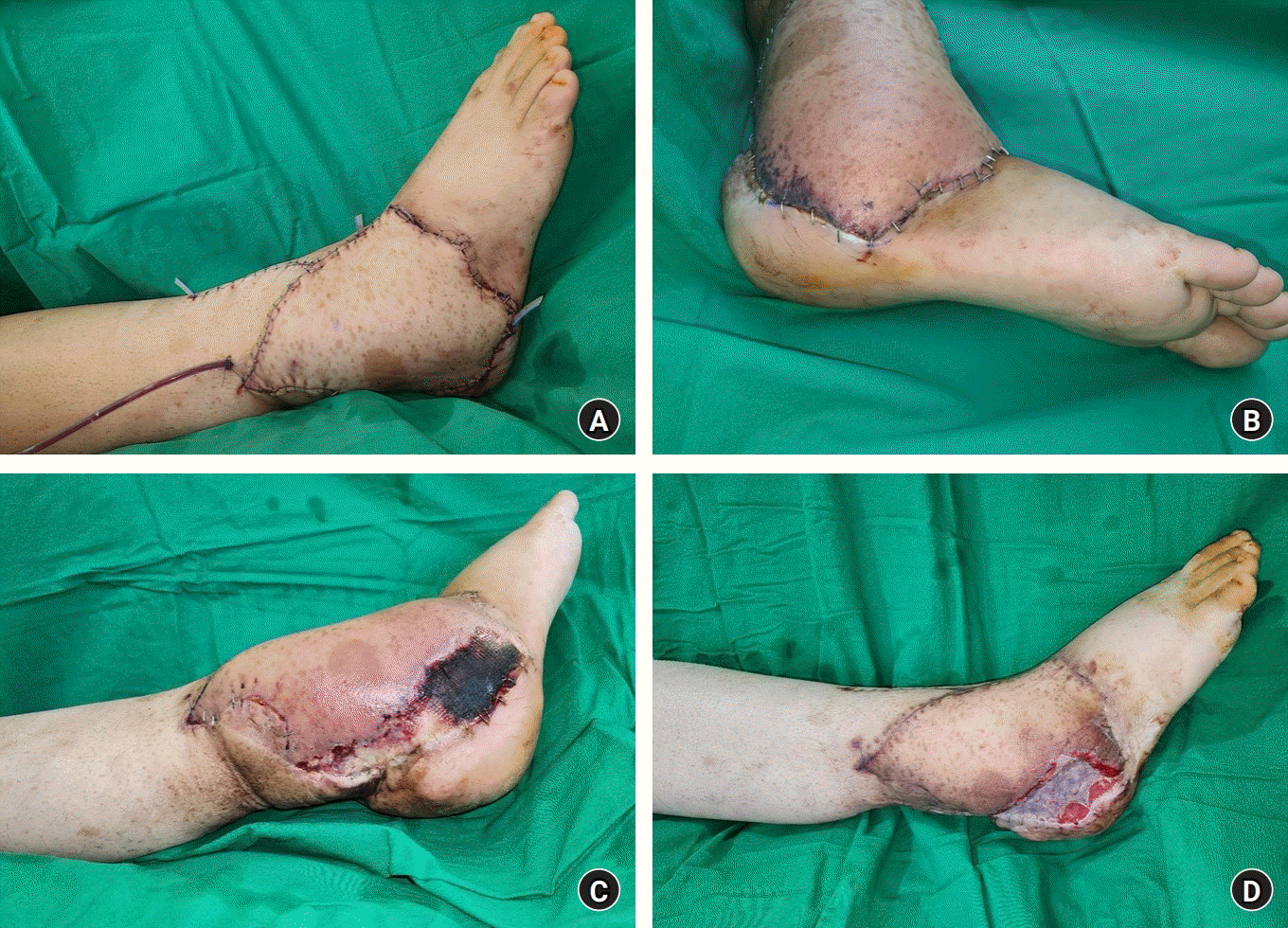
Fig. 4.
Sagittal view of postoperative T2-weighted magnetic resonance imaging revealed rapid growth of the known malignant peripheral nerve sheath tumor and the extent of local recurrence.
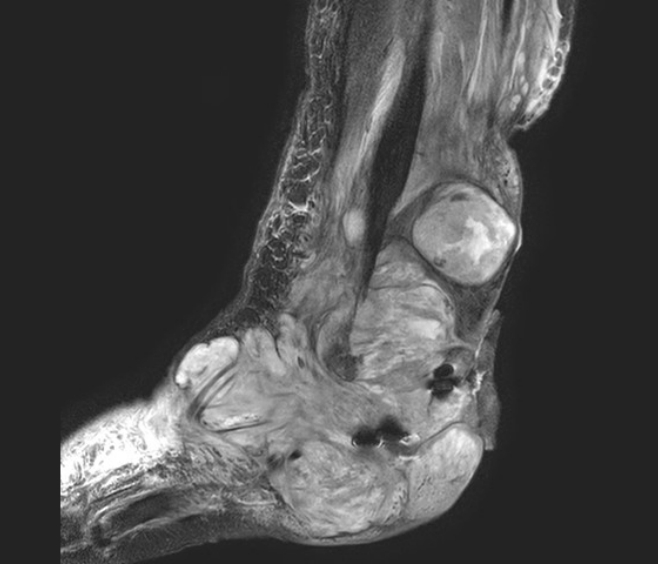
Fig. 5.
(A) The histologic image of the resected tumor revealed spindle cells with irregular contours and high mitotic activity, a characteristic of high-grade malignant peripheral nerve sheath tumor ( H&E stain, ×400). (B) Cross-sections of the resected tumor revealed a diffusely located tumor with necrosis, infiltrating the surrounding normal tissue.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download