Abstract
Ancient schwannomas are benign, long-standing schwannomas of the neural sheaths. This schwannoma subtype is characterized by cystic and hemorrhagic changes. These degenerative changes are thought to result from the tumor’s long-term progression and have the potential to be misdiagnosed as other soft tissue tumors or sarcomas. A 37-year-old female patient presented to our department with discomfort caused by a mass on the anterior side of her forearm. Approximately 2 weeks prior to her visit, an excisional biopsy was attempted under local anesthesia, but the patient experienced severe tingling sensations up to the wrist during surgery, preventing successful removal of the mass. Ultrasonography and magnetic resonance imaging (MRI) were performed for further differential diagnosis. An excisional biopsy was performed under general anesthesia, and histologic analysis revealed typical degenerative features of ancient schwannoma. A thorough examination, including MRI, is necessary to identify these tumors, prevent potential misdiagnosis, and ensure appropriate treatment.
Schwannomas, also known as neurilemmoma, are the most common benign tumors of the neural sheaths of the peripheral nerves [1]. Typically, a schwannoma is attached to the nerve from which it originates, does not cause pain, and grows slowly. When a schwannoma occurs along peripheral nerves, the differential diagnosis can be easily performed through clinical symptom assessment and imaging tests. Usually, it has a firm consistency and round shape and is freely movable from the surrounding soft tissues. In the early stages, it lacks symptoms except for the feeling of a lump. Pain or neurological symptoms are influenced mainly by the mass [2,3].
As one of the schwannoma subtypes, ancient schwannomas are characterized by cystic and hemorrhagic degeneration. The term “ancient schwannoma” describes a tumor that has undergone degenerative changes and is considered a rare condition resulting from long-term tumor progression [4,5]. Because it contains cystic areas, ancient schwannomas can be radiologically misdiagnosed as other tumor types.
In this report, we relate the case of an ancient schwannoma, which developed in the median nerve in the forearm, misdiagnosed as a dermoid cyst. We described the clinical presentation and the specific imaging, histology, and surgical findings.
The Institutional Review Board of CHA Gumi Medical Center (No. GM21-16) reviewed and approved this study and the informed consent was taken from the patient.
A 37-year-old female patient visited our department due to discomfort caused by a mass in the anterior side of the forearm. It had been about 12 years since she recognized a painless mass on her left forearm. There was no significant change in size during that time, but it had gradually increased over the last 3 to 4 months. There was no history of trauma or systemic disease. The discomfort she complained of was due to the gross mass and pain and tingling sensation accompanied by the recent increase in size. There were no other muscle weaknesses, sensory abnormalities, or functional problems. The mass was diagnosed at the previously visited clinics as a dermoid cyst with fluid and solid layer hetero-echogenicity under ultrasonographic examination (Fig. 1).
About 2 weeks before the patient visited our department. An excisional biopsy was attempted under local anesthesia. Still, the tingling sensation up to the wrist during surgery was so severe that the mass could not be removed. After suspending the attempt, the patient was referred to our hospital.
Upon physical examination, there was a previous surgical incision about 6 cm in length on the anterior side of the forearm. A round, firm, and mobile mass with a diameter of about 3 cm was observed on palpation.
Grayscale sonography of the mass was performed on a LOGIQ S8 (GE Healthcare, Chicago, IL, USA) with a 12-MHz transducer. The examination showed a large well-circumscribed mass (3.8×3.5 cm) that had a fluid-solid and was filled with heterogeneous echoic material (Fig. 2). The mass with a fluid-solid level appeared as a layering of serous fluid and tumor contents, shows moderate posterior acoustic enhancement. No vascularity was observed within the mass on Doppler sonography.
Magnetic resonance imaging (MRI) was performed for further differential diagnosis. MRI showed an ovoid-shaped 2.8×2.0×3.6 cm mass on the anterior side of the median nerve, with a well-defined boundary. MRI shows an enhanced, well-demarcated tumor in the left forearm. A well-encapsulated isointense lesion on the T1-weighted image. Cystic and necrotic areas have low signal intensity in the T1 sequences. A heterogeneous enhancement in the central mass with the entering-exiting nerve sign and split-fat sign on the T2 fat-suppression image. Cystic and necrotic areas have high signal intensity in the T2 sequences. In the cystic mass, a fluid-solid level is seen within the central tumorous lesion (Fig. 3). Furthermore, the entering-exiting nerve sign, split-fat sign, and the T2 fat-suppression image confirmed continuity between the tumor and peripheral nerve.
An excisional biopsy was performed under general anesthesia. An incision was made on the volar side of the forearm in the same way as the previous one at the other hospital. A well-encapsulated oval-shaped mass in an eccentric position along the median nerve was observed during surgery. The tumor was enucleated with care not to damage the median nerve.
On gross pathologic examination, the actual size of the resected tumor was about 2.7×2.0×3.5 cm, and it was a well-circumscribed yellowish oval solid mass containing a cystic lesion tilled with friable, tannish gray material. The inside matrix was relatively firm, red or yellow, and some myxoid changes and hematomas were observed (Fig. 4).
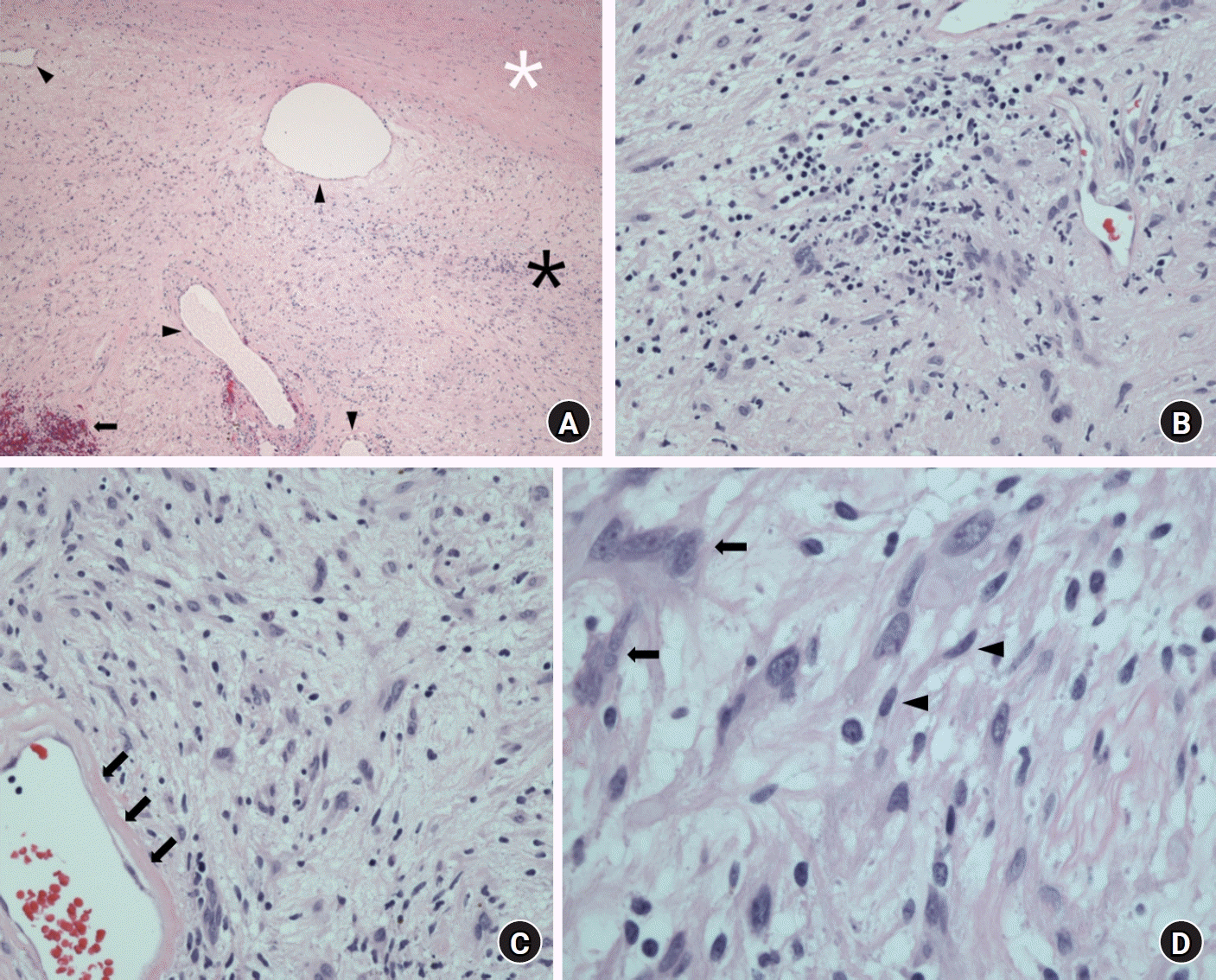
Histologic analysis revealed the typical finding of degenerative features of ancient schwannoma. On microscopic examination, alternating Antoni A (highly ordered cellularity) and Antoni B (hypocellular areas of spindle cell zones) were observed, and a necrotic lesion with multiple cystic areas filled with inflammatory cells and red blood cells was also seen. In regions of degenerative changes, there was extensive hemorrhaging, hyalinization, and myxoid changes. Focal nuclear atypia with no mitotic figures was observed together with typical neurogenic tumor cells as degenerative changes. Based on these histopathological features, the lesion was diagnosed as an ancient schwannoma (Fig. 5).
After surgery, there was no problem in the sensory and motor functions of the extremities, and the surgical wound was also clean. Furthermore, there was no discomfort at the operation site and no tumor recurrence at the outpatient follow-up 1 year after surgery.
Schwannomas are benign encapsulated tumors of nerve sheath origin that are most commonly found in the extremities. Typically, schwannomas have a slow growth pattern. Therefore, it does not cause pain in the early period and allows for the adaptation of nerve function to the pressure effects. Pain or neurological symptoms are influenced mainly by the mass [2]. Relevant to classical schwannomas, the term “ancient schwannoma” is used to describe a tumor that has undergone degenerative changes. It is an uncommon condition and is most common in elderly patients [4,5].
Ancient schwannomas may be misdiagnosed as other types of tumors, such as malignant fibrous histiocytoma, malignant peripheral nerve sheath, and synovial sarcoma, based on the radiologic findings of cystic degeneration. If the tumor has thick walls and central cystic areas, it should also be considered in the differential diagnosis.
In the present case, the patient did not clinically experience severe discomfort for a long time except for the feeling of a lump. Its location was superficial and easily palpable in the middle of the forearm. At the previous clinic visit, ultrasound was used as the first-line imaging method of choice for diagnosis. The findings of an intralesional heterogenic echogenic round mass with an air-fluid level and no connection to other soft tissue led to the diagnosis of a dermoid cyst. It is not easy to differentiate a schwannoma from a dermoid cyst because of the nature of the cystic component.
Ultrasound examination is a helpful diagnostic that reveals a mass’s cystic nature in clinics or at the bedside. It is a rapid, noninvasive, and low-cost procedure. Moreover, there is no need for exposure to ionizing radiation. However, since the ultrasound examination is an operator-dependent procedure, a suboptimal technique can also lead to misdiagnosis. According to a review by Yasumatsu et al. [6], ultrasound examination images of a schwannoma typically show a well-defined, hypoechoic, and primarily homogeneous ovoid (or round) solid mass with or without moderate posterior acoustic enhancement but rarely show heterogeneities and cystic changes inside the mass.
Although the previous physician thought the mass lesion was a dermoid cyst, clinical examination associated with the subsequently evaluated MRI features suggest a long-standing schwannoma with degenerative changes. MRI findings are related to the histological characteristics of schwannomas: Antoni A area, which contain cellular components with collagen, exhibit low signals in the T1 and T2 images; and Antoni B area, which contain myxoid matrix with water, produce low T1 signal intensity and high signal intensity in T2 images . A rim of low intensity surrounds the lesion corresponding to the epineural capsule. In our case, the finding of a fluid-solid level in the central area of the mass, which was seen in the previous ultrasonographic examination, represented tumor necrosis. The morphology and signal intensities reflect the histologic components of schwannomas and cystic and necrotic areas. Cystic and necrotic areas have low signal intensity in T1 sequences and high signal in T2 sequences. Heterogeneous signal areas combined with cystic formations are occasionally interpreted as aggressive features in many peripheral nerve sheath tumors or other high-grade sarcomas. Physicians should be aware of this issue and interpret the findings cautiously. The pathognomonic target sign of schwannoma was observed, and the continuity with peripheral nerve travel was also confirmed. Therefore, we could clinically diagnose this tumor as a schwannoma, omit the biopsy procedure, and perform a primary tumor excision. Marginal excision is the general surgical treatment for ganglion cysts and benign solid tumors [7]. Since schwannomas are well-encapsulated, it is almost always possible to enucleate and separate the tumor from the neural membrane. Enucleation using a microsurgical technique is the standard surgical procedure for a schwannoma. We dissect the nerve fascicles and successfully enucleate the whole tumor while preserving the nerve fascicles. As a result, nerve functions are preserved, and the patient did not exhibit sensory or motor deficits postoperatively.
Histopathologically, schwannomas are divided into the following variants of common, plexiform, cellular, epithelioid, and ancient schwannomas [8]. The ancient schwannoma is a rare variant characterized by degenerative changes and a diffuse hypocellular area. It was described first in 1951 by Ackerman and Taylor [9]. An ancient schwannoma has a true capsule composed of epineurium. The inner tumor mass generally displays a biphasic pattern with highly ordered areas. It is the pathologic hallmark of schwannomas, which presents as alternating patterns of Antoni A and B areas [1]. Densely packed Antoni A regions are cellular areas with spindle cells and peculiar linear arrangements of elongated tumor nuclei, whereas Antoni B areas are hypocellular areas in a myxoid background. Besides these classical pathologic features of conventional schwannomas, ancient schwannomas can have bizarre spindle cells and a large cystic myxomatous area, which are attributed to vascular insufficiency caused by the long-term progression of the tumor. Moreover, the tumor size itself has also been correlated with progressive degenerative features [8]. However, these additional features of degenerative atypia and cells with large and hyperchromatic nuclei can lead to diagnostic difficulties from their malignant counterpart [1,9]. Rockwell et al. [10] reported that the accurate differential diagnosis of schwannoma and other mass lesions was necessary because an incorrect preoperative diagnosis may lead to iatrogenic nerve resection and permanent neurologic deficits.
We presented a case of an ancient schwannoma with thick walls and cystic areas, which was initially misdiagnosed as a dermoid cyst. With long-standing progression and an increase in tumor size, schwannomas can undergo degenerative changes, including cyst formation, and show variable sonographic features. Even though ultrasound examination is a useful diagnostic tool for schwannoma as a first-line radiological investigation, thorough examinations, including MRI, are necessary to differentiate them from other tumor types and prevent a potential misdiagnosis. A proper diagnosis of the lesion should be established before surgery.
References
1. Weiss SW GJ, Enzinger FM. Enzinger and Weiss’s soft tissue tumours. 4th ed. St. Louis: Mosby;2001. p. 1111–208.
2. MacCollin M, Chiocca EA, Evans DG, et al. Diagnostic criteria for schwannomatosis. Neurology. 2005; 64:1838–45.

3. Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br. 2007; 89:382–7.
4. Hide IG, Baudouin CJ, Murray SA, Malcolm AJ. Giant ancient schwannoma of the pelvis. Skeletal Radiol. 2000; 29:538–42.

5. Schultz E, Sapan MR, McHeffey-Atkinson B, Naidich JB, Arlen M. Case report 872. “Ancient” schwannoma (degenerated neurilemoma). Skeletal Radiol. 1994; 23:593–5.
6. Yasumatsu R, Nakashima T, Miyazaki R, Segawa Y, Komune S. Diagnosis and management of extracranial head and neck schwannomas: a review of 27 cases. Int J Otolaryngol. 2013; 2013:973045.

7. Koga H, Matsumoto S, Manabe J, Tanizawa T, Kawaguchi N. Definition of the target sign and its use for the diagnosis of schwannomas. Clin Orthop Relat Res. 2007; 464:224–9.

8. Vilanova JR, Burgos-Bretones JJ, Alvarez JA, Rivera-Pomar JM. Benign schwannomas: a histopathological and morphometric study. J Pathol. 1982; 137:281–6.

Fig. 1.
Ultrasound image from the clinic the patient previously visited. The mass has a round, oval shape, a wall with a relatively clear border, and internal heterogeneity.
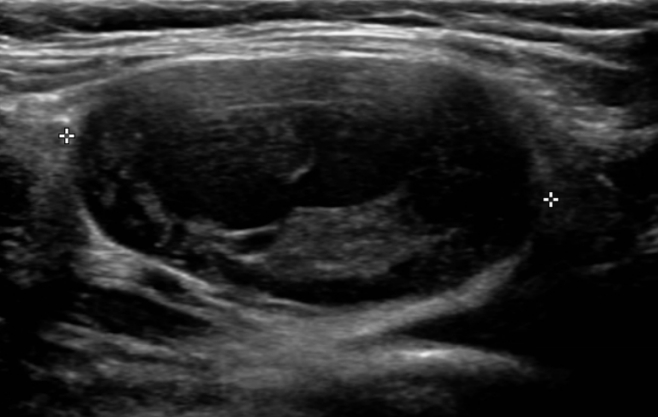
Fig. 2.
Ultrasound image from our hospital. The examination shows a large well-circumscribed mass (3.8×3.5 cm) with a fluid-solid or fluid-fluid level filled with heterogeneous echoic material.
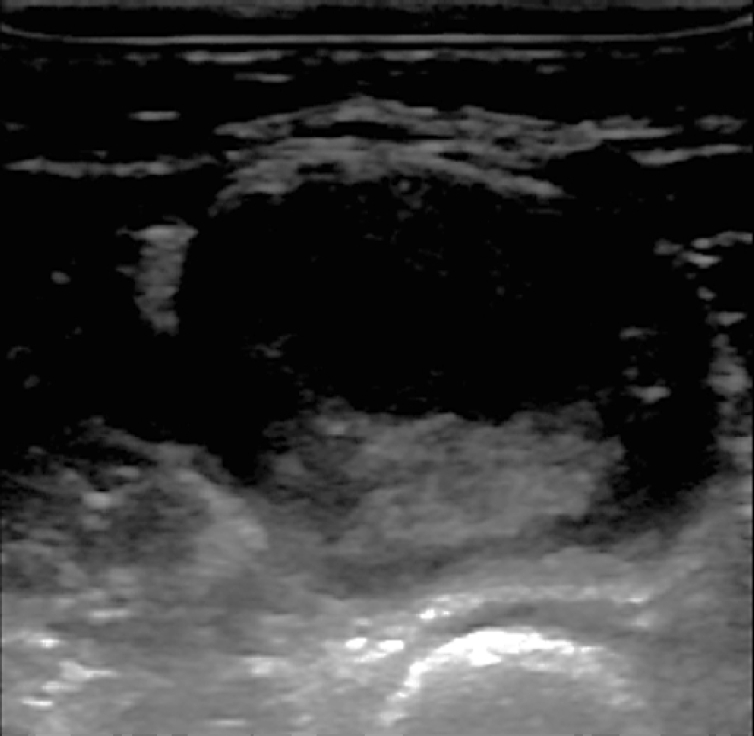
Fig. 3.
Magnetic resonance imaging shows an enhanced, well-demarcated tumor in the left forearm. (A) A well-encapsulated isointense lesion on a T1-weighted image. The cystic and necrotic areas have low signal intensity in the T1 sequences. (B) Heterogeneous enhancement in the central mass with the entering-exiting nerve sign and split-fat sign on a T2 fat-suppression image. The cystic and necrotic areas have high signal intensity in the T2 sequences. In the cystic mass, a fluid-solid level is seen within the central tumorous lesion.
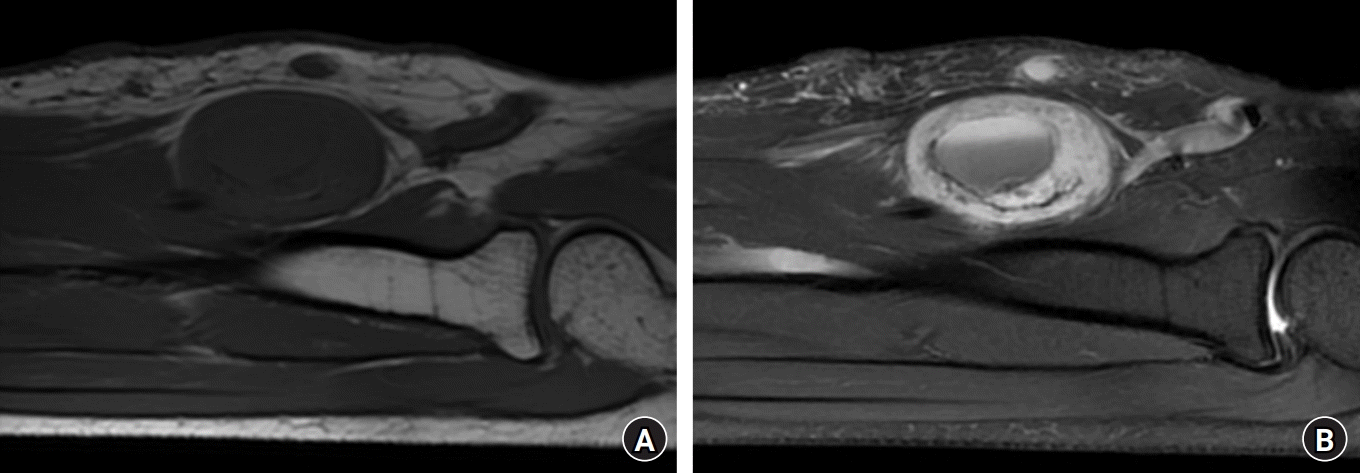
Fig. 4.
(A) A well-encapsulated mass anterior to the median nerve was observed. (B) The actual size of the resected tumor was 2.7×2.0×3.5 cm, and it was a firm and yellow oval with a clear border. (C) Macroscopic view of the enucleated tumor. The cut surface revealed myxomatous changes accompanied by cystic degeneration and hematomas.
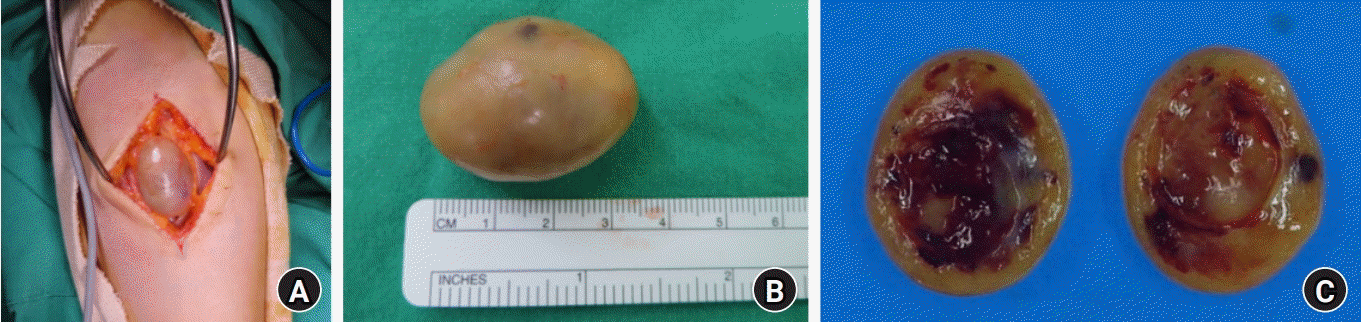
Fig. 5.
Microscopic findings. (A) In the low-magnification micrograph, alternating Antoni A (black asterisk) and Antoni B (white asterisk) patterns are seen. Several blood vessels (black arrowheads) and hemorrhages (black arrow) are observed as well (H&E stain, ×40). (B) Multiple inflammatory cells representing degenerative changes are observed (H&E stain, ×100). (C) The thickened blood vessel wall is hyalinized (black arrows) (H&E stain, ×200). (D) Degenerative atypical cells (black arrows) with typical neurogenic tumor cells (black arrowheads) are observed (H&E stain, ×400).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download