According to the American Diabetes Association (ADA), there are two types of diabetic neuropathies (DPN): typical and atypical forms. The typical DPN is a distal symmetric sensorimotor polyneuropathy (DSPN) which is length-dependent and considered the most common variety. DSPN is a chronic neurodegenerative disorder; up to 50% of cases may have no neuropathic symptoms. However, DSPN significantly contributes to foot ulcers as the disorder progresses, which can lead to amputation, falls, and fractures. Therefore, clinicians must diagnose DSPN as early as possible to start early intervention for proper long-term care [1].
To diagnose DPN, Diabetes Control and Complications Trial (DCCT) had proposed an electrodiagnosis protocol [2], but something else is needed for DSPN by definition [3]. Original electrodiagnostic protocol for DPN proposed by DCCT included the nerves in the upper and lower extremity. By ADA definition, DSPN involves the sensory nerves first and motor nerves later in the lower extremity, followed by the upper extremity nerves later [1,4]. Electrodiagnosis for most typical DSPN can be made with lower extremity nerve conduction studies, and upper extremity nerve conduction study may be redundant, may consider the tests in cases of absent responses from lower extremity nerves [4].
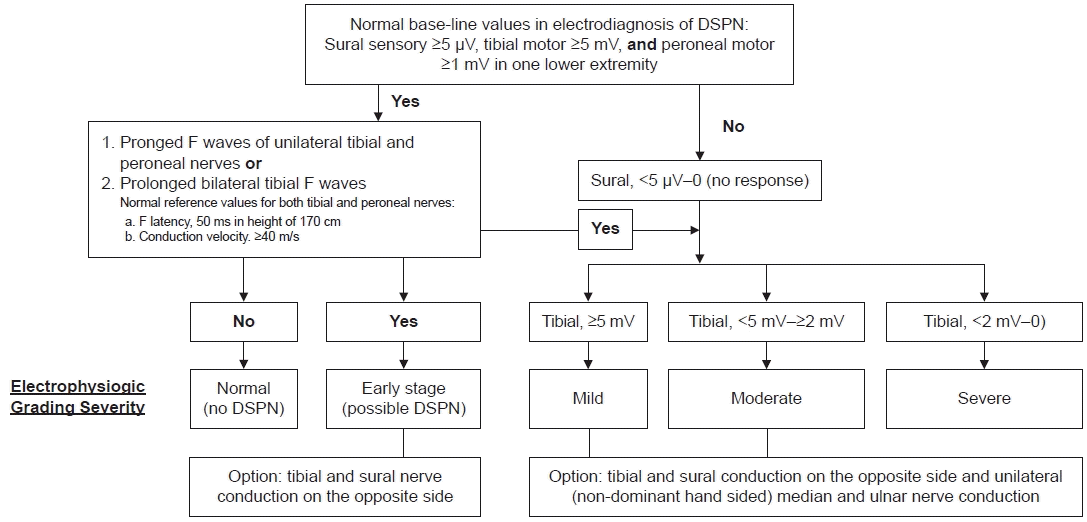
Diagnosing the DSPN, nerve conduction studies for three major nerves-sural sensory, tibial, and peroneal nerves unilaterally or bilaterally (probably optional) are enough [5]. Many electrodiagnostic studies for diagnosing DSPN have been tried to achieve more sensitive tests: tibial F latency tests were reported to be the most sensitive parameter [6].
To establish normal reference values, we conducted a retrospective medical record review of tibial F wave data from 145 patients. The study received approval from the institutional review board. The electrodiagnosis of diabetic DSPN, particularly in its early stage, can be determined by examining the minimal latencies of the tibial and peroneal nerves. Among patients with diabetes, the tibial F latency is considered the most sensitive parameter [6]. The peroneal F latency is similar to the tibial latency (Spearman correlation coefficient 0.88, p-value<0.001) in our study. The severity of the condition is assessed based on the amplitudes of the sural and tibial nerves. If the compound muscle action potential of the tibial nerve measures less than 2 mV (indicating severe grading, excluding severe lumbosacral radiculopathies), the action potentials of the sural or medial plantar nerves cannot be recorded (based on our unpublished data from 12 out of 219 diabetic patients). Additionally, these patients exhibit both neuropathic symptoms and neuropathic signs, such as the absence of response to pinprick and tuning fork vibration response.
Because no effective treatments specifically address peripheral nerve damage, it is extremely important to focus on prevention in diabetes care. It is also crucial to screen for symptoms and signs of diabetic DSPN in practice, as this can help identify the initial stages of nerve damage, allowing early intervention [1].
We propose this algorithm formulated in conjunction with previously reported protocols [2,7,8] and our data for the early detection or estimation of severity for diagnosing DSPN (Fig. 1). If there is no evoked response, especially from the tibial motor nerve, it is advisable to conduct a conduction study of the median and ulnar nerves in the upper extremity. This additional test will contribute to a more comprehensive evaluation of DSPN. Needle electromyography can also provide additional insights into the severity of DSPN or any other underlying neuromuscular disorders, like radiculopathy or other types of neuropathy.
Notes
REFERENCES
1. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017; 40:136–54.

2. Report and recommendations of the San Antonio conference on diabetic neuropathy. Consensus statement. Diabetes. 1988; 37:1000–4.
3. England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. Distal symmetrical polyneuropathy: definition for clinical research. Muscle Nerve. 2005; 31:113–23.

4. Perkins BA, Ngo M, Bril V. Symmetry of nerve conduction studies in different stages of diabetic polyneuropathy. Muscle Nerve. 2002; 25:212–7.

5. Tankisi H, Pugdahl K, Beniczky S, Andersen H, Fuglsang-Frederiksen A. Evidence-based recommendations for examination and diagnostic strategies of polyneuropathy electrodiagnosis. Clin Neurophysiol Pract. 2019; 4:214–22.

6. Andersen H, Stålberg E, Falck B. F-wave latency, the most sensitive nerve conduction parameter in patients with diabetes mellitus. Muscle Nerve. 1997; 20:1296–302.

7. Baba M, Suzuki C, Ogawa Y. Severity grading system of diabetic neuropathy in type-2 diabetes by nerve conduction study: five-year prospective study on occurrence of diabetic foot, macroangiopathic events, and eventual death. JPN J Clin Neurophysiol. 2018; 46:71–7.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download