Abstract
Attenuated portal vein (PV) flow is challenging in pediatric liver transplantation (LT) because it is unsuitable for classic end-to-end jump graft reconstruction from a small superior mesenteric vein (SMV). We thus introduce a novel technique of an end-to-side jump graft from SMV during pediatric LT using an adult partial liver graft. We successfully performed two cases of end-to-side retropancreatic jump graft using an iliac vein graft for PV reconstruction. One patient was a 2-year-old boy with hepatoblastoma and a Yerdel grade 3 PV thrombosis who underwent split LT. Another patient was an 8-month-old girl who had biliary atresia and PV hypoplasia with stenosis on the confluence level of the SMV; she underwent retransplantation because of graft failure related to PV thrombosis. After native PV was resected at the SMV confluence level, an end-to-side reconstruction was done from the proximal SMV to an interposition iliac vein. The interposition vein graft through posterior to the pancreas was obliquely anastomosed to the graft PV. There was no PV related complication during the follow-up period. Using a jump vascular graft in an end-to-side manner to connect the small native SMV and the large graft PV is a feasible treatment option in pediatric recipients with inadequate portal flow due to thrombosis or hypoplasia of the PV.
Conducting a liver transplantation (LT) in children with inadequate inflow due to portal vein (PV) hypoplasia (≤ 4 mm in diameter) or thrombosis is much more challenging than those in adults. These cases present with higher rates of complications, including twisting, thrombosis, and stenosis, which can lead to graft dysfunction from size mismatch; this is typically the result of a large-for-size graft. For this reason, PV complications in pediatric LT are common, with an incidence of 6% to 12% [1-3].
Various surgical techniques have been introduced to overcome PV issues in pediatric LT. For instance, vein graft interposition in cases of PV hypoplasia or thrombosis, as well as collateral interruption, have been shown to lead to improved outcomes [4,5]. Further, direct reconstruction by the ellipsoid technique [6] is a solution currently being applied for PV hypoplasia patients.
However, if there is stenosis at the confluence level of the superior mesenteric vein (SMV) and the splenic vein (SV), direct reconstruction becomes difficult. Moreover, in cases of PV thrombosis (PVT) related to tumorous conditions, such as hepatoblastoma, it is important to calculate sufficient margin for reconstruction. Thus, we should consider jump venous interposition reconstruction in those cases [7]. Considering interposition jump graft using a size-matched donor iliac vein (IV) to the graft left PV of a big-sized adult left liver for adequate inflow, the size of the native SMV was unsuitable.
Thus, we adopted and modified the pullout technique for reconstruction of a retropancreatic jump graft in an “end-to-side” manner to overcome the size-mismatch of small native SMV and a deceased adult donor IV.
A 2-year-old boy with hepatoblastoma was treated with chemotherapy (PRETEXT stage 4); the response was not positive (POSTTEXT stage 4), as his initial α-fetoprotein level was 1,063 ng/mL and increased to 13,348 ng/mL. He had a history of KBG syndrome and cardiac surgery for pulmonary atresia with ventricular septal defect and dynamic obstruction of right ventricle outflow tract. He was admitted for split LT for hepatoblastoma with a pediatric end-stage liver disease (PELD) score of 7. Pre-LT computed tomography (CT) scans showed diffuse tumor involvement of the whole liver and obliteration of intrahepatic portal venous channels and main PV. The gastric varices from the SMV drained to the inferior vena cava (IVC) (Fig. 1A). He received a split left lateral section graft from a deceased donor. PVT was determined to be Yerdel grade 3 [8], and extended to the confluence of the SMV and SV. The obliterated native PV was pulled out through the posterior aspect of the pancreas (Fig. 1B) [7], the native PV was resected, and an eversion thrombectomy was performed. There was no tumor involvement to thrombus and vascular resection margin. For the reconstruction of the PV, we performed retropancreatic jump vascular graft using the same donor-fresh IV. The IV was anastomosed in an end-to-side manner to the recipient SMV using a 6-0 PDS running suture under control of the inflows including the SMV and SV and the outflow, which is gastric varix. Upon completion of the anastomosis, the IV jump graft was passed posteriorly to the pancreas after measuring the proper length of the graft. Dextroplantation was performed. The left lateral section graft was positioned at the right side of the IVC [9], and the IV graft was anastomosed to the graft left PV in an oblique method. Cold ischemic time was 192 minutes, and warm ischemic time was 47 minutes. The total operation time was 705 minutes.
The patient stayed in the intensive care unit (ICU) for 5 days for cardiac monitoring. On postoperative day 20, he suffered from pneumonia and recovered well with antibiotic treatment. He was discharged on postoperative day 26. Follow-up CT scans (Fig. 1A) 3 months after LT showed intact flow, and his condition has remained good without any complication for 5 months as of this writing.
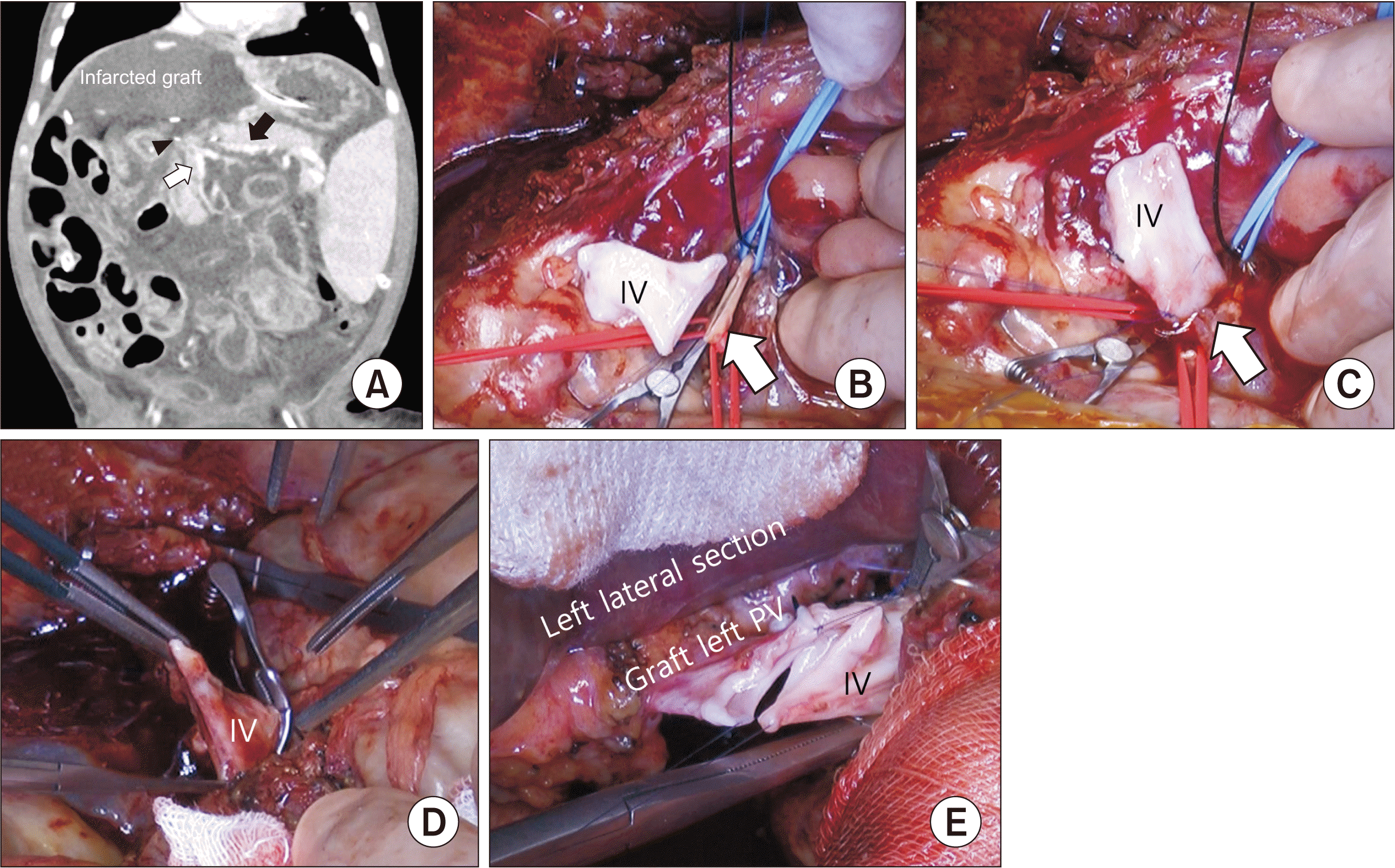
An 8-month-old girl received a Kasai operation to treat biliary atresia four months after birth. She underwent a deceased donor split LT 8 months after birth. Unfortunately, she suffered from early liver graft failure in postoperative day 9 due to PVT because significant stenosis just below the confluence level of the SMV and SV (Fig. 2A) was missed during the first LT. An emergency living donor LT from her father was thus performed. The orifice of the SMV was too small and sclerotic to provide enough inflow, and there was a huge size discrepancy between the IV for jump graft and the SMV orifice. Thus, the IV jump graft was anastomosed to the SMV in an end-to-side manner, and the PV was reconstructed to the graft in the same way (Fig. 2B–2E). Transplantation procedures were performed as described in the first case. The cold ischemic time was 61 minutes whereas the warm ischemic time was 33 minutes. The total operation time was 310 minutes.
The patient stayed in the ICU for 10 days. On postoperative day 14, there was anastomotic stricture of the bile duct, which was treated with percutaneous bile duct drainage. She was discharged on postoperative day 25. She has been doing well without any complication for 24 months as of this writing.
Non-tumoral PVT is observed in 5% to 26% of LT candidates [10]. With the introduction of new surgical technical options [11,12], PVT has not been considered an absolute contraindication of LT. Yerdel et al. [8] has proposed a PVT classification, which is graded by the extent and severity of PVT and plays an important role in determining surgery strategy. It is still difficult to perform LT in patients with PVT grade 3 or higher PVT. Thus, we used a “pull through” technique [7] to resect PVT completely, obtain a sufficiently large margin for reconstruction, and resect SMV thrombosis in this first case.
In our center, ellipsoid oblique or modified ellipsoid direct anastomosis is typically used in small recipients with PV hypoplasia [9]. However, in these particular cases, one patient needed PV resection to obtain tumor negative margin and the other showed sclerotic stenosis below the confluence of the SMV and SV. Thus, as mentioned above, the native PV stump was pulled out through the posterior tunnel of the pancreas and resected. The pullout technique [7] could provide a clear surgical field to evaluate this stenotic portion which was not detected during the first transplantation in case 2. In case 1, the stump of the SMV was very small, because of multiple draining veins to the pancreas, while in case 2, it was because of sclerosis of the confluence of the SMV and SV. Therefore, we could not anastomose the SMV to the graft PV in a classic end-to-end manner. Thus, we modified the SMV jump graft in an “end-to-side” manner. Then, after passing the graft through the retropancreatic space, anastomosis to the graft PV was performed in the usual manner because the size of the IV jump graft was well matched to the graft left PV.
PV stenosis is one of the most common complications in pediatric LT, with incidences ranging from 3% to 14%, and it is significantly higher than in adults [1,13-15]. PV stenosis could result in portal hypertension, paraesophageal varices, gastrointestinal bleeding, and even graft failure if left untreated [16]. In these two cases, because the vessel graft was anastomosed in an end-to-side manner to the small SMV, which could make it possible to have a sufficient diameter of an interposition vascular graft to prevent PV stenosis. We also obliquely anastomosed an interposition graft to the graft PV to secure a wide diameter [9].
In cases where a pediatric LT recipient has a high-grade PVT or sclerotic narrow confluence of the SMV and SV which does not allow for the native PV to be used, it is feasible to adopt an “end-to-side” retropancreatic jump graft to connect between the SMV and the graft PV. This helps to enable PV reconstruction and prevent PV stenosis, and is also used in cases of large left lateral section graft from an adult donor.
REFERENCES
1. Ueda M, Egawa H, Ogawa K, Uryuhara K, Fujimoto Y, Kasahara M, et al. 2005; Portal vein complications in the long-term course after pediatric living donor liver transplantation. Transplant Proc. 37:1138–1140. DOI: 10.1016/j.transproceed.2005.01.044. PMID: 15848648.
2. Yin C, Zhu ZJ, Wei L, Sun LY, Zhang HM, Wu HR. 2020; Risk factors for portal vein stenosis in pediatric liver transplantation. Clin Transplant. 34:e13992. DOI: 10.1111/ctr.13992. PMID: 32453915.
3. Jensen MK, Campbell KM, Alonso MH, Nathan JD, Ryckman FC, Tiao GM. 2013; Management and long-term consequences of portal vein thrombosis after liver transplantation in children. Liver Transpl. 19:315–321. DOI: 10.1002/lt.23583. PMID: 23495080.
4. Sakamoto S, Uchida H, Kitajima T, Shimizu S, Yoshimura S, Takeda M, et al. 2020; The outcomes of portal vein reconstruction with vein graft interposition in pediatric liver transplantation for small children with biliary atresia. Transplantation. 104:90–96. DOI: 10.1097/TP.0000000000002793. PMID: 31205260.
5. Sakamoto S, Sasaki K, Kitajima T, Hirata Y, Narumoto S, Kazemi K, et al. 2018; A novel technique for collateral interruption to maximize portal venous flow in pediatric liver transplantation. Liver Transpl. 24:969–973. DOI: 10.1002/lt.25016. PMID: 29351365.
6. Yi NJ, Lee JM, Kim H, Choi Y, Lee HW, Kim HY, et al. 2016; Simple ellipsoid reconstruction technique for a hypoplastic portal vein during pediatric liver transplantation. Liver Transpl. 22:854–858. DOI: 10.1002/lt.24445. PMID: 27028426.
7. Kasahara M, Sasaki K, Uchida H, Hirata Y, Takeda M, Fukuda A, et al. 2018; Novel technique for pediatric living donor liver transplantation in patients with portal vein obstruction: the "pullout technique". Pediatr Transplant. 22:e13297. DOI: 10.1111/petr.13297. PMID: 30280455.
8. Yerdel MA, Gunson B, Mirza D, Karayalçin K, Olliff S, Buckels J, et al. 2000; Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation. 69:1873–1881. DOI: 10.1097/00007890-200005150-00023. PMID: 10830225.
9. Ahn SW, Yi NJ, Kim HC, Ahn HY, Hong SK, Lee JM, et al. 2021; Dextroplantation of left liver graft in infants. Liver Transpl. 27:222–230. DOI: 10.1002/lt.25883. PMID: 32897624.
10. Rodríguez-Castro KI, Porte RJ, Nadal E, Germani G, Burra P, Senzolo M. 2012; Management of nonneoplastic portal vein thrombosis in the setting of liver transplantation: a systematic review. Transplantation. 94:1145–1153. DOI: 10.1097/TP.0b013e31826e8e53. PMID: 23128996.
11. Lerut JP, Mazza D, van Leeuw V, Laterre PF, Donataccio M, de Ville de Goyet J, et al. 1997; Adult liver transplantation and abnormalities of splanchnic veins: experience in 53 patients. Transpl Int. 10:125–132. DOI: 10.1111/j.1432-2277.1997.tb00554.x. PMID: 9089998.
12. Selvaggi G, Weppler D, Nishida S, Moon J, Levi D, Kato T, et al. 2007; Ten-year experience in porto-caval hemitransposition for liver transplantation in the presence of portal vein thrombosis. Am J Transplant. 7:454–460. DOI: 10.1111/j.1600-6143.2006.01649.x. PMID: 17229075.
13. Yabuta M, Shibata T, Shibata T, Shinozuka K, Isoda H, Okamoto S, et al. 2014; Long-term outcome of percutaneous transhepatic balloon angioplasty for portal vein stenosis after pediatric living donor liver transplantation: a single institute's experience. J Vasc Interv Radiol. 25:1406–1412. DOI: 10.1016/j.jvir.2014.03.034. PMID: 24854391.
14. Vasavada B, Chen CL. 2015; Vascular complications in biliary atresia patients undergoing living donor liver transplantation: analysis of 110 patients over 10 years. J Indian Assoc Pediatr Surg. 20:121–126. DOI: 10.4103/0971-9261.154651. PMID: 26166981. PMCID: PMC4481622.
15. Patel R, Mahaveer J, Tahir N, Rajwal S, McClean P, Patel JV. 2018; Outcomes of percutaneous portal vein intervention in a single UK paediatric liver transplantation programme. Cardiovasc Intervent Radiol. 41:96–103. DOI: 10.1007/s00270-017-1792-0. PMID: 28913651. PMCID: PMC5735201.
16. Alfares BA, Bokkers RPH, Verkade HJ, Dierckx RAJO, Gupte G, Franchi-Abella S, et al. 2021; Portal vein obstruction after pediatric liver transplantation: a systematic review of current treatment strategies. Transplant Rev (Orlando). 35:100630. DOI: 10.1016/j.trre.2021.100630. PMID: 34107368.
Fig. 1
Reconstruction process of the end-to-side portal vein (PV) and superior mesenteric vein (SMV) reconstruction in retransplantation with PV obliteration. (A) Pre- and post-transplant computed tomography (CT) scans of a hepatoblastoma patient. In the pre-transplant CT scans, it could be seen that PV (black arrowhead) was obliterated, and SMV (white arrow) was drained to gastric varix (white arrowhead). After transplant, recipient main PV and graft left PV (black arrowhead) were well-preserved in 3-month CT scans. (B) Obliterated portal vein and collaterals. Main portal vein stump was obliterated and collaterals to the gastric vein through pancreas were developed.
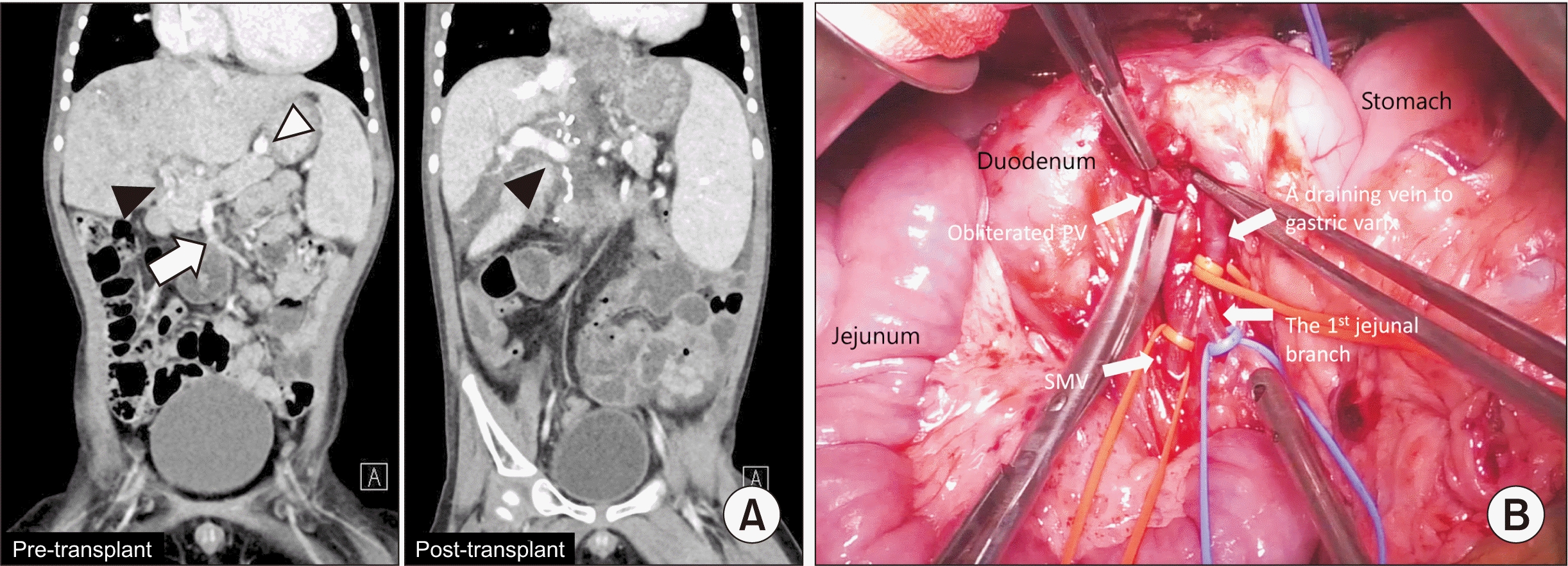
Fig. 2
Reconstruction process of the end-to-side portal vein (PV) and superior mesenteric vein (SMV) reconstruction in retransplantation with PV thrombosis. (A) Computed tomography (CT) scans before second liver transplantation. Left lateral section graft showed infarction because of severe PV (black arrowhead) stenosis at the confluence level. SMV (white arrow) was not dilated without collaterals. Black arrow: splenic vein. (B–E) Reconstruction process of the end-to-side PV and SMV reconstruction using an interposition jump graft. (B) The end of an iliac vein (IV) was approximated to the side of the SMV (white arrow) and temporally clamped. (C) The end-to-side reconstruction was done between the IV and SMV (white arrow). (D) The IV was passed through the retropancreatic path and reclamped for reconstruction to the graft PV. (E) The left PV of the left lateral section graft was anastomosed to a jump interposition IV graft using an oblique method.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download