Abstract
Hepatic arterioportal fistulae are abnormal communications between the hepatic artery and portal vein. They are reported to be congenital or acquired secondary to trauma, iatrogenic procedures, hepatic cirrhosis, and hepatocellular carcinoma, but less likely to occur spontaneously. Extrahepatic portal venous obstruction (EHPVO) can lead to pre-hepatic portal hypertension. A spontaneous superimposed hepatic arterioportal fistula can lead to pre-sinusoidal portal hypertension, further exacerbating its physiology. This report describes a young woman with long-standing EHPVO presenting with repeated upper gastrointestinal variceal bleeding and symptomatic hypersplenism. Computed tomography scan demonstrated a cavernous transformation of the portal vein and a macroscopic hepatic arterioportal fistula between the left hepatic artery and portal vein collateral in the central liver. The hepatic arterioportal fistula was associated with a flow-related left hepatic artery aneurysm and a portal venous collateral aneurysm proximal and distal to the fistula, respectively. Endovascular coiling was performed for the hepatic arterioportal fistula, followed by proximal splenorenal shunt procedure. This case illustrates an uncommon association of a spontaneous hepatic arterioportal fistula with EHPVO and the utility of a combined endovascular and surgical approach for managing multifactorial non-cirrhotic portal hypertension in such patients.
Extrahepatic portal venous obstruction (EHPVO) is a common cause of non-cirrhotic portal hypertension (NCPH) and variceal bleeding in developing countries [1,2]. Sequels of long-standing EHPVO include the development of portosystemic collaterals, a cavernous transformation of the portal vein, ectopic varices leading to gastrointestinal bleeding and portal cavernous cholangiopathy (PCC), splenomegaly with hypersplenism, growth retardation, and functional hepatic compromise [3,4]. Hepatic arterioportal fistulae (HAPF) are uncommon vascular lesions that can develop due to abnormal communication between the hepatic artery and portal vein. HAPF might be congenital or secondary to iatrogenic causes (e.g., post liver biopsy, transhepatic biliary drainage, surgery, and post-liver transplant), trauma, hepatic cirrhosis, and hepatic neoplasms [5,6]. Macroscopic HAPFs represent high-flow vascular lesions in which hepatic artery blood flow bypasses hepatic sinusoids to drain into the portal circulation, leading to pre-sinusoidal portal hypertension [5]. Thus, the mechanism of portal hypertension in HAPF (pre-sinusoidal) differs from that in EHPVO (pre-hepatic in origin), which can have management implications [1]. Rarely, HAPF may develop on a background of EHPVO and contribute to the underlying portal hypertensive physiology. This case reports a HAPF developing spontaneously in a patient with EHPVO and discusses clinical presentation, management, and implications of HAPF associated with EHPVO.
A 20-year-old woman initially presented with an index upper gastrointestinal (UGI) bleed (four episodes of hematemesis with ~500 mL of blood in each episode) four years ago. UGI endoscopy revealed two columns of small oesophageal varices. Multiphasic computed tomography (CT) performed at the index presentation four years ago demonstrated the sequel of EHPVO with thrombosed extrahepatic portal vein, portal cavernoma, and multiple portosystemic venous collaterals. The splenic vein and superior mesenteric veins were patent. Hepatic artery vasculature was unremarkable. Subsequently, she again presented with three episodes of hematemesis followed by three episodes of melena, symptomatic splenomegaly, and asymptomatic PCC. There was no history of trauma, liver biopsy, surgery, or percutaneous biliary drainage in the interval duration. The patient did not undergo any specific treatments for portal hypertension or portal vein obliteration either during this time.
At the current presentation, her blood tests results indicated anemia (hemoglobin: 7.2 g/dL), leucocytopenia (leucocyte counts: 1,800/µL), thrombocytopenia (platelets: 40,000/µL) with reticulocytosis (reticulocytes: 8.3%), and anisocytosis, suggesting hypersplenism. Liver function tests demonstrated borderline elevated serum aspartate transaminase level at 48.2 U/L (normal: < 35 U/L), normal serum alkaline transaminase level at 30.9 U/L (normal: < 35 U/L), and normal serum alkaline phosphatase level at 57 IU/L (normal range: 30–120 IU/L). She had mild direct hyperbilirubinemia, total serum bilirubin level of 3.06 mg/dL (normal range: 0.3–1.2 mg/dL) with direct bilirubin level of 0.51 mg/dL (normal range: < 0.2 mg/dL). She was negative for hepatitis B virus, hepatitis C virus, and HIV serology. Repeat UGI endoscopy showed four columns of large esophageal varices extending to lesser curvature with a red color sign (Sarin Grade 1 gastro-esophageal varices) [7], which were endoscopically ligated.
Hepatic Doppler sonography showed non-cirrhotic liver, hepatosplenomegaly, and portal cavernoma. The largest of these portal venous collaterals showed low-velocity hepatopetal flow with a peak systolic velocity of ~9.5 cm/sec and loss of normal respiratory/cardiac phasicity. Another portal venous collateral showed aneurysmal dilatation with to-and-fro flow. The splenic vein was dilated with increased hepatopetal flow (peak systolic velocity 24 cm/sec). Hepatic arteries were not well evaluated on Doppler.
Subsequent multiphasic CT again showed sequel of EHPVO with cavernous transformation of extrahepatic and proximal intrahepatic portal veins and multiple portosystemic collaterals in pericholecystic, pericholedochal, perigastric, periesophageal, perisplenic regions and submucosal esophageal varices. On the arterial phase, there was a 1.1 cm × 1.0 cm aneurysm arising from a branch of the left hepatic artery at the porta hepatis (Fig. 1A, 1B, 2). Adjacent to the left hepatic artery aneurysm, a branch directly communicated with a portal vein collateral with early opacification of the portal venous system in the arterial phase suggestive of HAPF (Fig. 1A, 1B, 2). There was also a 2.6 cm × 2.5 cm portal venous collateral aneurysm adjacent to the HAPF (Fig. 1C). The splenic artery was ectatic with a small distal splenic artery aneurysm. There was a replaced right hepatic artery arising from the superior mesenteric artery and coursing posterior and superior to the HAPF (Fig. 2). The spleen was enlarged measuring 19 cm in length (Fig. 1D) while the liver was small in size (10 cm), showing morphologic changes of chronic liver disease (widened fissures, severe left lobe atrophy and caudate lobe hypertrophy). There was mild central intrahepatic and extrahepatic biliary ductal dilation consistent with PCC.
Based on the spectrum of imaging findings, an imaging diagnosis of flow-related left hepatic artery aneurysm with a macroscopic HAPF between left hepatic artery and portal vein collateral was made. As the HAPF had developed in the absence of any known causes (trauma, cirrhosis, malignancy or iatrogenic procedures), it was diagnosed as a spontaneous HAPF on a background of EHPVO. The portal venous collateral aneurysm distal to the HAPF was a consequence of direct shunting of high-flow hepatic arterial blood across the HAPF into the portal venous collateral.
The patient received endovascular management of HAPF. After a left radial artery access with a 5 French (5F) short sheath, a 5F Headhunter H1 catheter was placed in the common hepatic artery after hooking the celiac artery. Common hepatic artery angiogram demonstrated the left hepatic artery aneurysm and a branch shunting blood into a portal venous channel inferiorly consistent with HAPF (Fig. 3A). Further selective catheterization of the left hepatic artery was performed using a microcatheter (Progreat; Terumo Interventional Systems) (Fig. 3B). A pushable microcoil (18-5-2, Micronester; Cook Medicals) was deployed just across the neck of the aneurysm. Post-embolization angiogram demonstration successful closure of the HAPF (Fig. 3C).
A few days later, the patient underwent a proximal splenorenal shunt (PSRS) procedure with splenectomy and surgical shunt between splenic vein and left renal vein. Portal vein pressure (measured in the omental vein) was 34 cm of water and 20 cm of water before and after shunt creation, respectively. Intraoperatively liver surface was normal. Surgical liver biopsy specimen demonstrated maintained lobular architecture with preserved reticulin framework consistent with EHPVO. Portal tracts showed mild lympho-mononuclear inflammatory infiltrate and mild ductular proliferation in the form of a few angulated bile ductules at the periphery of portal tracts suggestive of early cholangiopathy.
On one-month follow-up, her hemogram showed improved parameters compared to pre-treatment results. Repeat blood tests showed improved hemoglobin level at 9.6 g/dL, improved leucocyte counts at 8,670 cells/µL, improved counts of platelets at 150,000 cells/µL, and resolved reticulocytosis (reticulocytes: 0%). Liver function tests were like prior. However, her serum bilirubin had completely normalized, with total serum bilirubin level at 0.38 mg/dL (normal range: 0.3–1.2 mg/dL) and direct bilirubin level at 0.08 mg/dL (normal range: < 0.2 mg/dL).
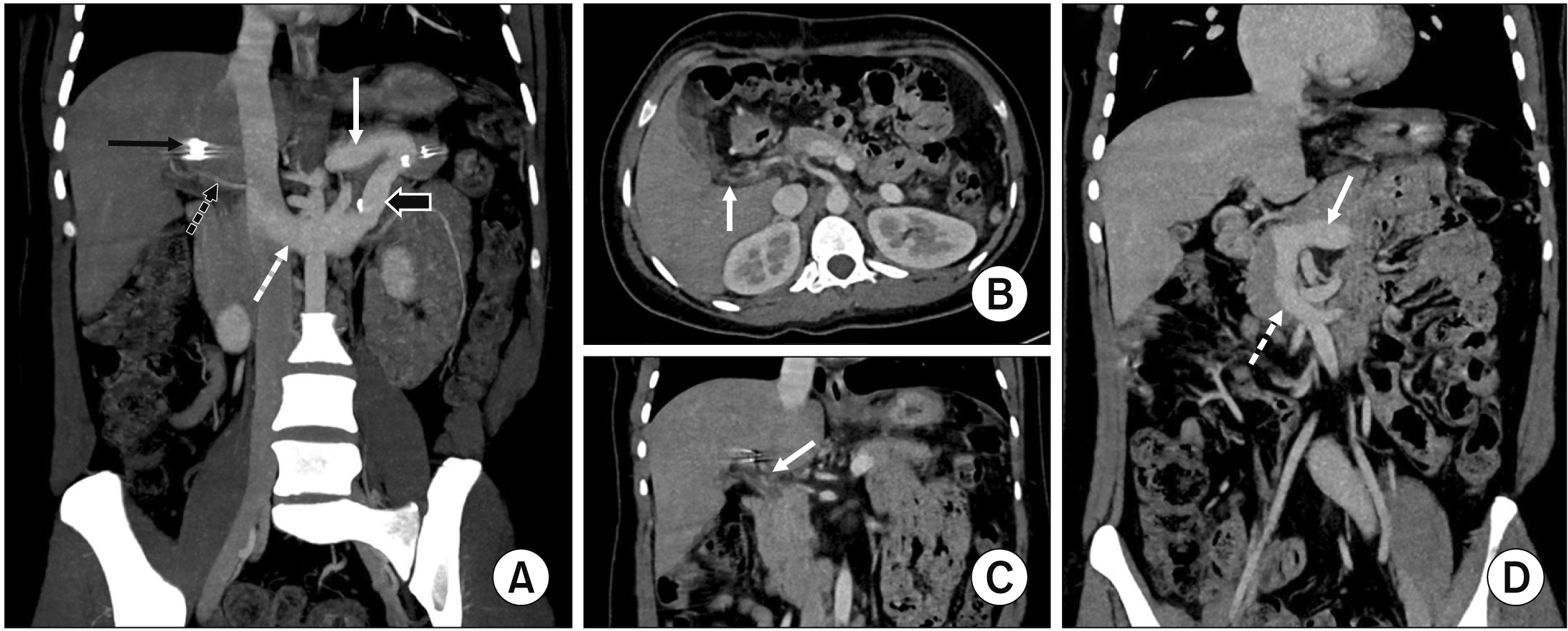
On subsequent follow-up visits, she was symptomatically better without showing any recurrent UGI bleeding or new symptoms. Hemogram was within normal limits (hemoglobin: 10.9 g/dL, leucocyte counts: 5,400 cells/µL, platelets: 355,000 cells/µL). Her serum bilirubin was also normal, showing total serum bilirubin level of 0.6 mg/dL (normal range: 0.3–1.2 mg/dL) and direct bilirubin level at 0.12 mg/dL (normal range: < 0.2 mg/dL). A repeat triple-phase CT at three-month follow-up showed endovascular coil in adequate position without recurrent HAPF and resolution of portal cavernous malformation without any residual portal vein collateral aneurysm (Fig. 4A–4C). The PSRS was widely patent (Fig. 4A). There was substantial regression of all previously seen portosystemic collaterals (Fig. 4D) and improvement in PCC with resolved central intrahepatic biliary ductal dilation.
EHPVO is a common cause of NCPH in developing Asian countries. EHPVO is a childhood vascular disorder characterized by thrombosis of the extrahepatic portal vein with or without the involvement of intrahepatic portal veins, superior mesenteric veins, and splenic veins [3]. EHPVO can occur due to multiple etiologies, which can be broadly grouped into inherited or acquired prothrombotic states, portal vein injuries (e.g., umbilical vein catheterization and abdominal sepsis in neonates, post-liver transplant or post-surgical injury), and local inflammation (e.g., pancreatitis or liver abscess). Additional etiologies in adult women include pregnancy and oral contraceptive pills [8]. Despite extensive workup, EHPVO can be idiopathic in more than half of cases [8]. In the present case, the etiology of EHPVO was presumed to be due to early childhood infection, the most common etiology in our patient population. Irrespective of the specific etiology of EHPVO, all cases of EHPVO are characterized by cavernous transformation of portal vein secondary to the opening of portoportal collaterals to preserve hepatopetal flow [3]. Despite the formation of portal cavernoma as early as three weeks after EHPVO, portal cavernoma is eventually ineffective in shunting the entire splenic-mesenteric flow to the liver, leading to pre-hepatic portal hypertension and secondary development of portosystemic collaterals, spontaneous portosystemic shunts, and ectopic varices to bypass the liver [8]. Over time, the hepatic arterial inflow also increases as an additional measure to compensate for inadequate portal hepatopetal flow, leading to an overall hyperdynamic circulation and enlargement of the hepatic artery (with/without celiac trunk enlargement) [9]. While liver parenchymal functions remain preserved in EHPVO until the very late-stage of the disease, massive splenomegaly, hypersplenism, variceal bleeding, growth retardation, and PCC are common clinical features of EHPVO [3].
HAPF is an uncommon cause of NCPH. It is characterized by anatomic or functional connections between the hepatic artery and portal vein branches. In HAPF, hepatic arterial flow bypasses hepatic sinusoids to drain into portal vein, leading to “intrahepatic, pre-sinusoidal” portal hypertension [1,8]. Multiple causes of HAPF have been described in the literature. These are rarely congenital. More commonly, they are secondary to trauma, iatrogenic procedures (e.g., liver biopsy, transhepatic biliary drainage, surgery, liver transplant), cirrhosis, and hepatic neoplasms (most commonly hepatocellular carcinoma) [5,6,10,11]. Spontaneous development of HAPF is uncommon. HAPF has been broadly described as type 1 (small peripheral intrahepatic type), type 2 (large central HAPF as seen in this case); and type 3 (diffuse congenital intrahepatic type) [5,12]. On triple phasic CT or MRI, central HAPF can be visualized as arterial enhancement of main portal vein before opacification of splenic or superior mesenteric vein. Large peripheral HAPFs are visualized as early arterial enhancement of peripheral portal vessels before enhancement of main portal vein. Small peripheral HAPF or diffuse HAPF can appear as sharply marginated, wedge-shaped areas of arterial hyperenhancement that can be isoenhancing to the background liver on the portal venous phase, termed as transient hepatic attenuation difference on CT or transient hepatic enhancement difference on CT or MRI [11-13].
Various mechanisms for the development of HAPF have been described, such as transplexal (i.e., arterial blood flow shunted through peribilary plexus in the setting of portal vein obstruction), transvasal (i.e., hepatic artery vasa vasorum shunting blood to recanalize a thrombus in portal vein), transtumoral (i.e., fistulous communications across hypervascular tumor vessels), transsinusoidsal (i.e., hepatic artery blood retrogradely draining into portal venule through hepatic sinusoids due to increased hepatic venous pressure as seen in Budd-Chiari syndrome), and macroscopic (i.e., direct communication between hepatic artery and portal vein commonly seen after penetrating liver injury) [11,12]. The HAPF in our case might have more than one of these mechanisms, given the sequel of prior portal vein obstruction, presence of adjacent hepatic artery aneurysm, macroscopic size of the HAPF, and associated PCC.
To the best of our knowledge, imaging appearances of HAPF developing spontaneously in EHPVO have not been described previously in the literature. In a retrospective analysis of 97 cases of HAPF, two patients had “portal spongiform transformation.” However, further details of imaging appearances and causes of HAPF in these two patients were not described [6]. Of these two patients, one was treated with transcatheter embolization while the other patient did not receive any specific treatment. Our case necessitated immediate intervention due to the presence of a macroscopic, central HAPF and the presence of an adjacent hepatic artery aneurysm. Potential consequences of untreated HAPF in this case include further exacerbation of the underlying portal hypertension, hepatoportal sclerosis due to local hepatic steal phenomenon, and risk of left hepatic artery aneurysm rupture [14].
Endovascular treatment of HAPF is now considered the preferred first-line treatment of HAPF due to its minimally invasive nature [15,16]. Both central HAPF and peripheral HAPF can be closed with transcatheter embolization. Choices of embolization agents include coils (most preferred), vascular plugs, detachable balloons, liquid agents like onyx and cyanoacrylate glue, and stent grafts [16]. As part of the embolization procedure, it is necessary to go as close to the HAPF as possible to avoid non-selective embolization and take care of any collateral circulation that could develop after hepatic artery embolization. In the present case, endovascular embolization with coils allowed a single-step solution for obliteration of both left hepatic artery aneurysm and closure of the HAPF. However, surgery was still indicated for definitive treatment of EHPVO-related portal hypertension and variceal bleed [17-19]. In the present case, a PSRS was performed as the patient had symptomatic splenomegaly and hypersplenism. Despite being a non-selective shunt, PSRS is often considered the first-line surgical option in younger patients with gastro-esophageal varices refractory to endoscopic treatment, showing acceptable long-term outcomes [8,18]. In our patient, follow-up imaging also demonstrated expected regression of portal cavernoma, portosystemic collaterals, and varices secondary to diversion of portal blood through the splenorenal shunt.
In conclusion, EHPVO and HAPF are two distinct vascular causes of NCPH. Rarely a HAPF may develop in EHPVO with portal cavernoma further exacerbating portal hypertension. While transcatheter embolization is preferred for HAPF, such patients may also need shunt surgeries for underlying EHPVO. Thus, a combined endovascular and surgical approach may be useful for managing NCPH in such patients.
REFERENCES
1. Sarin SK, Kumar A. 2006; Noncirrhotic portal hypertension. Clin Liver Dis. 10:627–651. DOI: 10.1016/j.cld.2006.08.021. PMID: 17162232.
2. Poddar U, Thapa BR, Rao KL, Singh K. 2008; Etiological spectrum of esophageal varices due to portal hypertension in Indian children: is it different from the West? J Gastroenterol Hepatol. 23:1354–1357. DOI: 10.1111/j.1440-1746.2007.05102.x. PMID: 17683492.
3. Sarin SK, Agarwal SR. 2002; Extrahepatic portal vein obstruction. Semin Liver Dis. 22:43–58. DOI: 10.1055/s-2002-23206. PMID: 11928078.
4. Sarin SK, Sollano JD, Chawla YK, Amarapurkar D, Hamid S, Hashizume M, et al. 2006; Consensus on extra-hepatic portal vein obstruction. Liver Int. 26:512–519. DOI: 10.1111/j.1478-3231.2006.01269.x. PMID: 16761994.
5. Guzman EA, McCahill LE, Rogers FB. 2006; Arterioportal fistulas: introduction of a novel classification with therapeutic implications. J Gastrointest Surg. 10:543–550. DOI: 10.1016/j.gassur.2005.06.022. PMID: 16627220.
6. Cao B, Tian K, Zhou H, Li C, Liu D, Tan Y. 2022; Hepatic arterioportal fistulas: a retrospective analysis of 97 cases. J Clin Transl Hepatol. 10:620–626. DOI: 10.14218/JCTH.2021.00100. PMID: 36062281. PMCID: PMC9396316.
7. Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. 1992; Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 16:1343–1349. DOI: 10.1002/hep.1840160607. PMID: 1446890.
8. Khanna R, Sarin SK. 2014; Non-cirrhotic portal hypertension - diagnosis and management. J Hepatol. 60:421–441. DOI: 10.1016/j.jhep.2013.08.013. PMID: 23978714.
9. Rajesh S, Mukund A, Sureka B, Bansal K, Ronot M, Arora A. 2018; Non-cirrhotic portal hypertension: an imaging review. Abdom Radiol (NY). 43:1991–2010. DOI: 10.1007/s00261-018-1570-8. PMID: 29564495.
10. Vauthey JN, Tomczak RJ, Helmberger T, Gertsch P, Forsmark C, Caridi J, et al. 1997; The arterioportal fistula syndrome: clinicopathologic features, diagnosis, and therapy. Gastroenterology. 113:1390–1401. DOI: 10.1053/gast.1997.v113.pm9322535. PMID: 9322535.
11. Choi BI, Chung JW, Itai Y, Matsui O, Han JK, Han MC. 1999; Hepatic abnormalities related to blood flow: evaluation with dual-phase helical CT. Abdom Imaging. 24:340–356. DOI: 10.1007/s002619900512. PMID: 10390555.
12. Wang Q, Koniaris LG, Milgrom DP, Patel A, Hu M, Cui E, et al. 2019; CT and MRI imaging and interpretation of hepatic arterioportal shunts. Transl Gastroenterol Hepatol. 4:34. DOI: 10.21037/tgh.2019.05.05. PMID: 31231701. PMCID: PMC6556701.
13. Itai Y, Moss AA, Goldberg HI. 1982; Transient hepatic attenuation difference of lobar or segmental distribution detected by dynamic computed tomography. Radiology. 144:835–839. DOI: 10.1148/radiology.144.4.6287520. PMID: 6287520.
14. Liu GF, Wang XZ, Luo XF. 2021; Simultaneous embolization of a spontaneous porto-systemic shunt and intrahepatic arterioportal fistula: a case report. World J Clin Cases. 9:9577–9583. DOI: 10.12998/wjcc.v9.i31.9577. PMID: 34877293. PMCID: PMC8610867.
15. Hirakawa M, Nishie A, Asayama Y, Ishigami K, Ushijima Y, Fujita N, et al. 2013; Clinical outcomes of symptomatic arterioportal fistulas after transcatheter arterial embolization. World J Radiol. 5:33–40. DOI: 10.4329/wjr.v5.i2.33. PMID: 23494252. PMCID: PMC3596609.
16. Chaudry G, Lillis AP, Shaikh R, Padua HM, Chewning RH, Alomari AI. 2018; Endovascular treatment of congenital arterioportal fistulas. Cardiovasc Intervent Radiol. 41:1021–1028. DOI: 10.1007/s00270-018-1924-1. PMID: 29511867.
17. Lal R, Sarma MS, Gupta MK. 2017; Extrahepatic portal venous obstruction: what should be the mainstay of treatment? Indian J Pediatr. 84:691–699. DOI: 10.1007/s12098-017-2390-5. PMID: 28612224.
18. Poddar U, Borkar V. 2011; Management of extra hepatic portal venous obstruction (EHPVO): current strategies. Trop Gastroenterol. 32:94–102. PMID: 21922871.
19. Rajalingam R, Javed A, Sharma D, Sakhuja P, Singh S, Nag HH, et al. 2012; Management of hypersplenism in non-cirrhotic portal hypertension: a surgical series. Hepatobiliary Pancreat Dis Int. 11:165–171. DOI: 10.1016/S1499-3872(12)60143-X. PMID: 22484585.
Fig. 1
(A) Coronal maximum intensity projection image from arterial phase computed tomography (CT) demonstrating a left hepatic artery aneurysm (solid white arrow) with a large branch leading to early arterial opacification of portal venous collateral (dashed white arrow) suggestive of hepatic arterioportal fistula (HAPF). (B) Axial thin section arterial phase CT at the level of portal venous collateral demonstrating early arterial opacification of portal venous collateral (arrow) before opacification of splenic and superior mesenteric veins consistent with HAPF. (C) Axial thin section venous phase CT at the level of portal venous collateral (same as B) demonstrating a portal collateral vein aneurysm (solid white arrow) distal to the HAPF. Also shown is a cavernous transformation of extrahepatic portal vein (dashed white arrow) consistent with extrahepatic portal venous obstruction (EHPVO). (D) Coronal multiplanar reformat image from venous phase CT demonstrating additional findings of EHPVO such as portosystemic collaterals (black arrow) and massive splenomegaly. The superior mesenteric vein (dashed white arrow) and splenic vein (solid white arrow) were patent in this case.
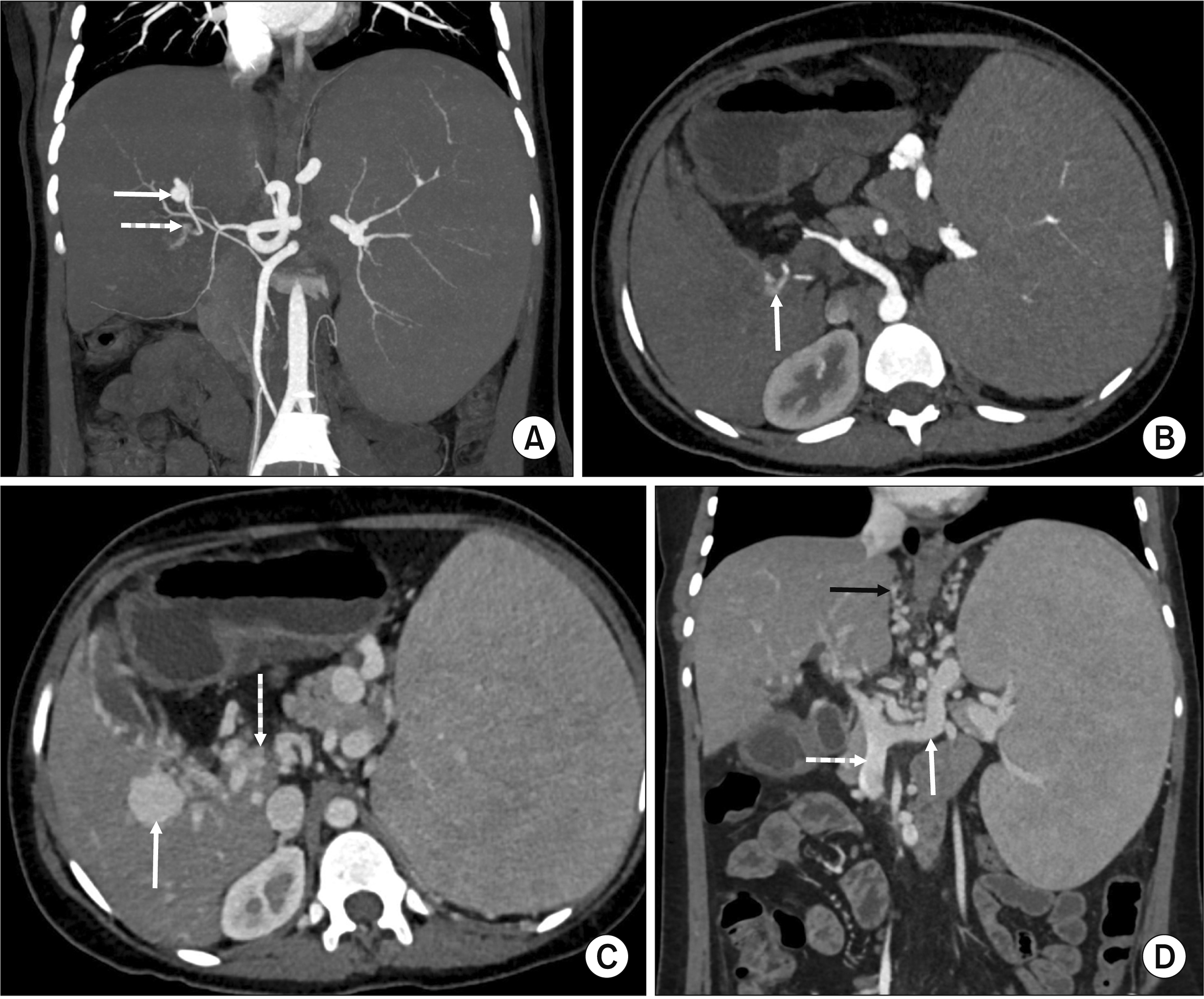
Fig. 2
Volume rendered technique image from arterial phase computed tomography demonstrating the spectrum of arterial findings, including left hepatic artery (LHA) aneurysm and macroscopic hepatic arterioportal fistula (HAPF) with arterial opacification of portal vein collateral. Also note the replaced right hepatic artery (RHA) arising from superior mesenteric artery (SMA) coursing posterior to the HAPF and the markedly ectatic splenic artery with small aneurysm.
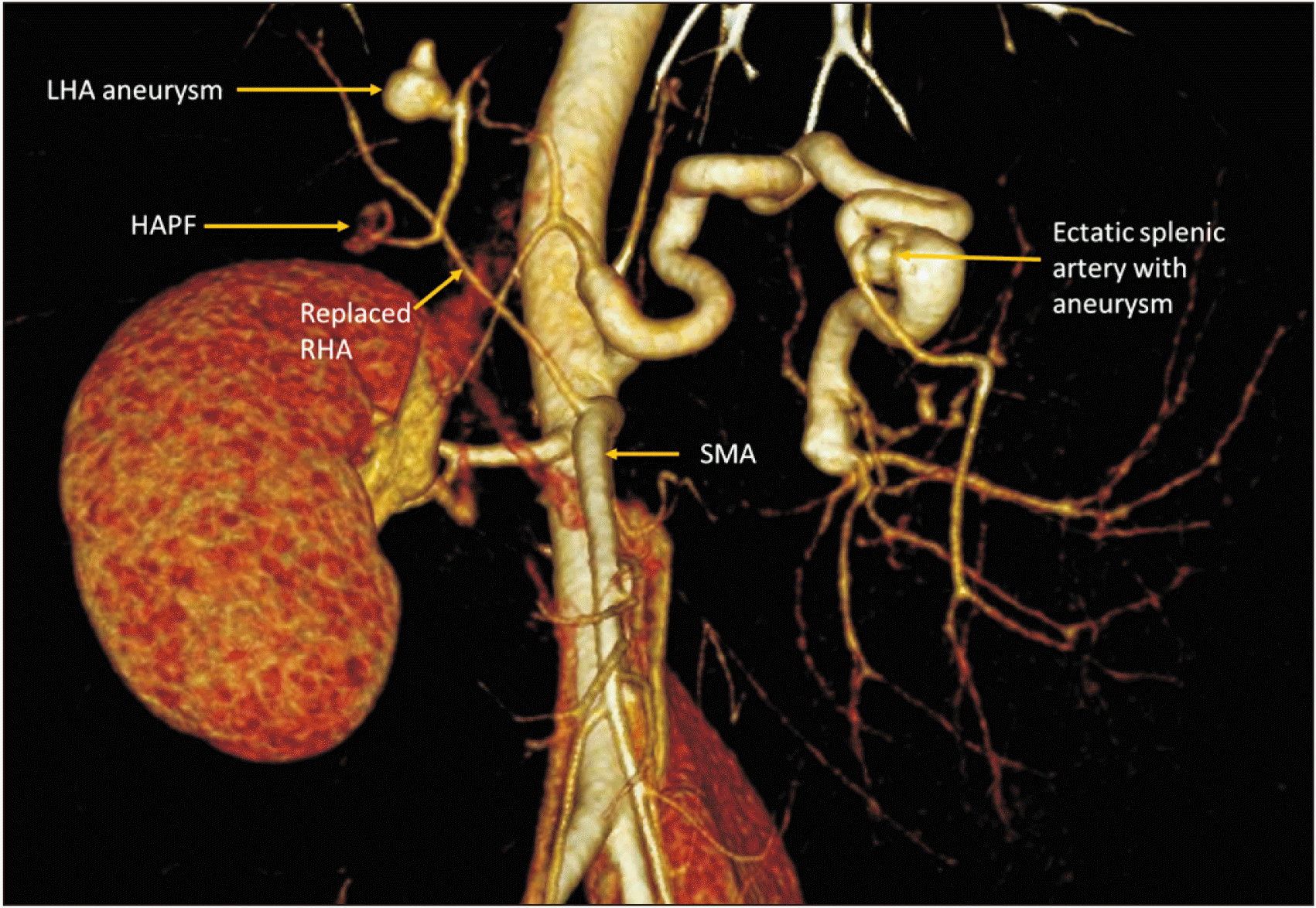
Fig. 3
Selected digital subtraction angiogram images demonstrating endovascular management of hepatic arterioportal fistula (HAPF). Pre-embolization images from common hepatic artery angiogram (A) and left hepatic artery angiogram (B) demonstrating the left hepatic artery aneurysm (solid black arrow in A), shunting of arterial blood into a portal venous channel (dashed black arrows in A, B), and the macroscopic fistulous connection between left hepatic artery and the portal venous collateral (solid black arrow in B). (C) Post- embolization image from left hepatic artery angiogram showing complete obliteration of both left hepatic artery aneurysm and HAPF with no residual flow.
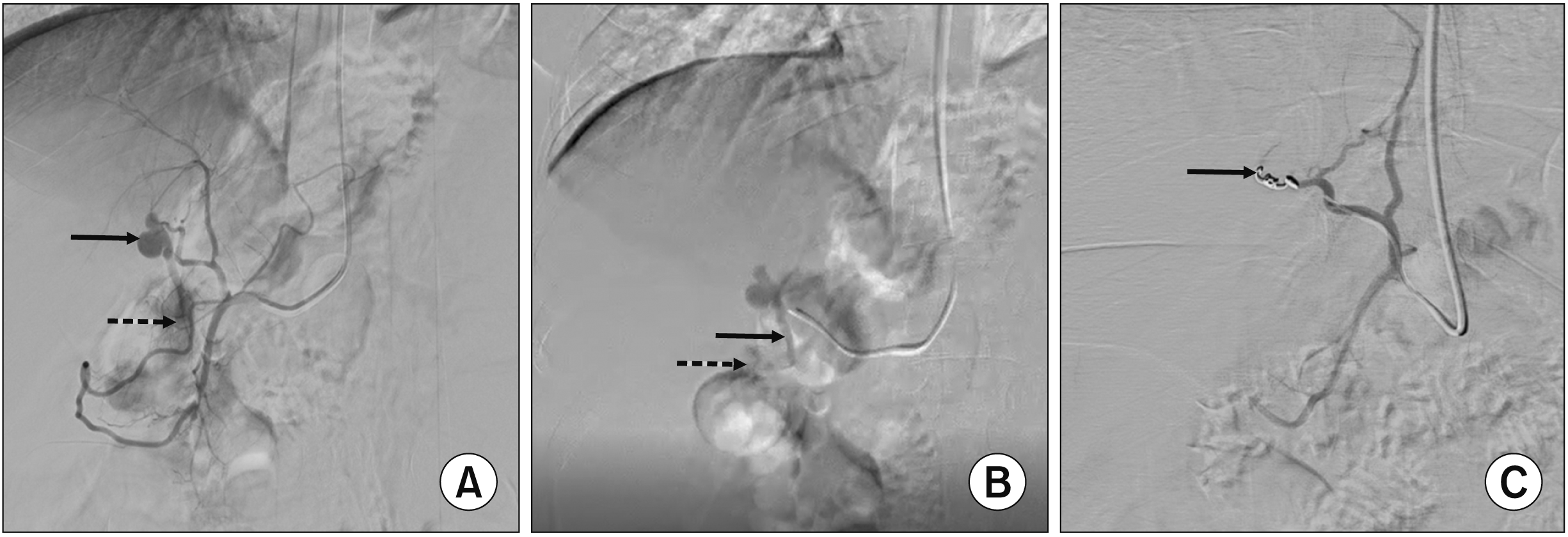
Fig. 4
(A) Coronal maximum intensity projection image from portal venous phase computed tomography (CT) demonstrating endovascular coil in left hepatic artery (solid black arrow). The splenorenal shunt is patent (black arrow with white outline). Proximal splenic vein (solid white arrow) and left renal vein (dashed white arrow) draining into the inferior vena cava are expected to be prominent. The replaced right hepatic artery is denoted by a dashed black arrow. Axial (B) and coronal (C) thin section portal venous phase CT at the level of porta demonstrating resolution of portal cavernoma without any residual portal vein collateral aneurysm (white arrows). The peribiliary venous plexus has also regressed with resolved central intrahepatic biliary dilation. (D) Coronal multiplanar reformat image from venous phase CT demonstrating expected changes of proximal splenorenal shunt with splenectomy, resolution of portosystemic collaterals and dilation of splenic vein (solid white arrow), and superior mesenteric vein (dashed white arrow) due to diversion of portal circulation.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download