MATERIALS AND METHODS
After obtaining data use agreement and institutional review board approval (MHS.2020.006), the 2014–2016 National Cancer Database (NCDB) was queried to identify all patients diagnosed with non-metastatic pancreatic adenocarcinoma who underwent pancreaticoduodenectomy. The study period began in 2010, the year that the NCDB Participant User File data item “rx_hosp_surg_appr_2010” was first made available. It documented surgical approach (robotic assisted, robotic converted to open, laparoscopic, laparoscopic converted to open, and open approach) in order to monitor patterns and trends in the adoption and utilization of minimally invasive surgical techniques. Patients with pancreatic adenocarcinoma were identified based on theInternational Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histology codes. ICD-O-3 histology codes used to select pancreatic adenocarcinoma included code 8140 (adenocarcinoma, not otherwise specified) and code 8500 (infiltrating ductal carcinoma, not otherwise specified). To establish a homogeneous cohort, patients with other histologic variants were excluded from analysis.
Patient characteristics such as age, race, gender, annual income, insurance status, Charlson-Deyo score, and year of diagnosis were obtained from the database. Surgical approach, surgical margins, total lymph node yield, positive lymph node yield, histology, tumor size, tumor grade, carbohydrate antigen 19-9 (CA19-9), and pathologic stage were similarly extracted from the database. Patients were then stratified into three groups based on surgical approach and need to convert to open: MI-PD, CO-PD, and O-PD. The MI-PD group was then further stratified into robotic pancreaticoduodenectomy (R-PD) and laparoscopic pancreaticoduodenectomy (L-PD) approach subgroups or robotic converted to open (R-CO) and laparoscopic converted to open (L-CO) subgroups.
Case-volume per year was calculated using case and facility ID and the year of diagnosis. The volume of patients treated at each facility was obtained by calculating the mean annual volume. The mean annual volume was estimated using the number of cases reported by a facility divided by the number of years in which a facility contributed to the report of cases. The number of cases was not simply divided by the number of years under study (2010–2016) since not all hospitals contributed to the report of cases for every year under study. Case-volume per year was then grouped into quartiles. The highest quartile was defined as “very high volume” and the lowest quartile was defined as “low volume” while the second and third quartiles were defined as “intermediate volume” and “high volume,” respectively.
Statistical analysis
Primary endpoints of this study were completeness of MI-PD and overall survival (OS). Secondary endpoints were surgical margin status, number of lymph nodes examined, hospital LOS, conversion status, 30-day mortality, 90-day mortality, and long-term survival (defined as having lived 60 months or longer after diagnosis).
Baseline characteristics were compared among the three groups (MI-PD, CO-PD, and O-PD) or five groups (R-PD, R-CO, L-PD, L-CO, O-PD) using one-way analysis of variance (ANOVA) for continuous variables and Pearson’s chi-square, or Fisher’s exact test where appropriate for categorical variables. Multivariable logistic regression modeling was used to examine binary outcomes such as 30-day mortality, 90-day mortality, conversion status, and long-term survival. All multivariable models except for the multivariable model assessing the association of conversion status with case-volume per year quartiles were adjusted for age, gender, race, insurance status, treatment facility type, Charlson-Deyo comorbidity score, case-volume per year quartiles, CA19-9, and tumor size. Multivariable models assessing the association of conversion status with case-volume per year quartiles were adjusted for the aforementioned control variables except for case-volume per year quartile which served as an independent variable.
Kaplan-Meier (KM) method with log-rank test was used to compare and estimate OS rates among the three groups as well as between case-volume per year quartiles. All OS analyses were performed after excluding patients who died within 30 days of their surgeries. Statistical significance was set at p < 0.05. All analyses were conducted using Stata/SE 15.
Go to :

RESULTS
Of 17,570 patients who underwent pancreaticoduodenectomy, 2,191 (12.5%) underwent MI-PD (2.3% R-PD and 10.2% L-PD), 734 (4.2%) underwent CO-PD (0.4% R-CO and 3.8% L-CO), and 14,645 (83.4%) underwent O-PD (
Table 1). Baseline demographics were similar among the cohorts with a median age of 67 years. Approximately 51% of patients were males. Patients who underwent MI-PD were more likely to be diagnosed between 2014–2016, while those in CO-PD and O-PD groups were more likely to be diagnosed from 2010-2013 (
p < 0.001). All races were more likely to have received an O-PD. The median income quartile and insurance status were not statistically different between groups. The Mean Crowfly for the MI-PD (82.8 ± 252.9) or CO-PD (71.4 ± 210.2) was higher than that for O-PD (57.4 ± 154.3) (
p < 0.001). The Charlson-Deyo score did not differ significantly among groups. Most patients had surgeries at an academic/research center with high (9.71–19) or very high (19.29–72.57) case-volume per year.
Table 1
Baseline characteristics of study population (n = 17,570)
|
MI-PD (%) |
CO-PD (%) |
O-PD (%) |
p-value |
|
Number (%) |
2,191 (12.5) |
734 (4.2) |
14,645 (83.4) |
- |
|
Median age (range), yr |
67.0 (29–90) |
67.5 (34–90) |
67.0 (24–90) |
|
|
Sex |
|
|
|
0.870 |
|
Male |
1,128 (51.5) |
383 (52.2) |
7,504 (51.2) |
|
|
Female |
1,063 (48.5) |
351 (47.8) |
7,141 (48.8) |
|
|
Race |
|
|
|
0.014*
|
|
White |
1,889 (86.6) |
632 (87.2) |
12,528 (86.3) |
|
|
Black |
199 (9.1) |
58 (8.0) |
1,491 (10.3) |
|
|
Asian |
59 (2.7) |
19 (2.6) |
345 (2.4) |
|
|
Other |
34 (1.6) |
16 (2.2) |
161 (1.1) |
|
|
Insurance Status |
|
|
|
0.089 |
|
Uninsured |
39 (1.8) |
- |
349 (2.4) |
|
|
Private Insurance |
816 (37.6) |
258 (35.4) |
5,266 (36.3) |
|
|
Medicaid |
108 (5.0) |
43 (5.9) |
786 (5.4) |
|
|
Medicare |
1,175 (54.2) |
414 (56.9) |
7,912 (54.6) |
|
|
Other Government |
30 (91.4) |
- |
185 (1.3) |
|
|
Median income quartiles (US $) |
|
|
|
0.172 |
|
< 40,227 |
329 (15.2) |
104 (14.3) |
2,469 (17.1) |
|
|
40,227–50,353 |
484 (22.3) |
161 (22.1) |
3,223 (22.3) |
|
|
50,354–63,332 |
508 (23.4) |
181 (24.9) |
3,334 (23.0) |
|
|
≥ 63,333 |
848 (39.1) |
282 (38.7) |
5,444 (37.6) |
|
|
No high school degree (%) |
|
|
|
< 0.001*
|
|
≥ 17.6 |
361 (16.6) |
102 (14.0) |
2,805 (19.4) |
|
|
10.9–17.5 |
530 (24.4) |
185 (25.4) |
3,747 (25.9) |
|
|
6.3–10.8 |
671 (30.9) |
225 (30.9) |
4,156 (28.7) |
|
|
< 6.3 |
611 (28.1) |
216 (30.0) |
3,787 (26.1) |
|
|
Crowfly |
82.8 ± 252.9 |
71.4 ± 210.2 |
57.4 ± 154.3 |
< 0.001*
|
|
Charlson-Deyo score |
|
|
|
0.141 |
|
0 |
1,381 (63.0) |
433 (59.0) |
9,364 (63.9) |
|
|
1 |
597 (27.3) |
232 (31.6) |
3,949 (27.0) |
|
|
2 |
149 (6.8) |
51 (7.0) |
944 (6.5) |
|
|
≥ 3 |
64 (2.9) |
18 (2.5) |
388 (2.7) |
|
|
Facility/cancer program type |
|
|
|
< 0.001*
|
|
Community |
25 (1.2) |
15 (2.1) |
221 (1.5) |
|
|
Comprehensive community |
370 (17.0) |
155 (21.3) |
3,303 (22.7) |
|
|
Academic/research |
1,505 (69.2) |
454 (62.5) |
9,004 (61.8) |
|
|
Integrated network |
274 (12.6) |
103 (14.2) |
2,034 (14.0) |
|
|
Case-volume per year quartiles |
|
|
|
< 0.001*
|
|
Low (≤ 3.40) |
287 (13.1) |
122 (16.6) |
2,566 (17.5) |
|
|
Intermediate (3.43–9.67) |
506 (23.1) |
143 (19.5) |
3,946 (26.9) |
|
|
High (9.71–19.00) |
595 (27.2) |
219 (29.8) |
4,180 (28.5) |
|
|
Very high (19.29–72.57) |
803 (36.7) |
250 (34.1) |
3,953 (27.0) |
|

Tumor characteristics were similar among groups (
Table 2). The majority of patients were Stage 2 (87.7% MI-PD, 87.9% CO-PD, 86.7% O-PD) with a tumor size ≥ 3 cm (56.0% MI-PD, 58.6% CO-PD, 57.0% O-PD) and lymphovascular invasion involving one or more positive lymph nodes. Tumors were mostly grades II and III. CA19-9 was greater than or equal to 98.9 U/mL in 54.4% of the MI-PD, 60.9% of patients in the CO-PD group and 54.5% of the O-PD group.
Table 2
Staging and tumor characteristics
|
MI-PD (%) |
CO-PD (%) |
O-PD (%) |
p-value |
|
Pathologic stage |
|
|
|
0.277 |
|
0 |
- |
- |
51 (0.4) |
|
|
1 |
181 (8.6) |
52 (7.3) |
1,200 (8.6) |
|
|
2 |
1,854 (87.7) |
625 (87.9) |
12,162 (86.7) |
|
|
3 |
31 (1.5) |
18 (2.5) |
316 (2.3) |
|
|
4 |
43 (2.0) |
15 (2.1) |
300 (2.1) |
|
|
Tumor size |
|
|
|
0.652 |
|
< 3 cm |
929 (43.9) |
296 (41.4) |
6,115 (42.9) |
|
|
≥ 3 cm |
1,184 (56.0) |
419 (58.6) |
8,118 (57.0) |
|
|
Microscopic focus |
- |
- |
14 (0.1) |
|
|
Grade |
|
|
|
0.001*
|
|
Well differentiated |
171 (7.8) |
42 (5.7) |
1,244 (8.5) |
|
|
Moderately differentiated |
1,026 (46.8) |
342 (46.6) |
6,980 (47.7) |
|
|
Poorly differentiated |
695 (31.7) |
234 (31.9) |
4,742 (32.4) |
|
|
Undifferentiated |
20 (0.9) |
- |
112 (0.8) |
|
|
Lymphovascular invasion |
|
|
|
0.036*
|
|
Present |
1,056 (54.0) |
382 (58.1) |
6,798 (53.1) |
|
|
Not present |
898 (46.0) |
276 (42.0) |
6,013 (46.9) |
|
|
Regional lymph node positivity |
|
|
|
0.640 |
|
Positive |
1,489 (68.1) |
506 (69.1) |
9,972 (68.2) |
|
|
Negative |
670 (30.6) |
222 (30.3) |
4,478 (30.6) |
|
|
No node examined |
27 (1.2) |
- |
164 (1.1) |
|
|
CA19-9 |
|
|
|
< 0.001*
|
|
0.0 U/mL |
- |
- |
18 (0.1) |
|
|
0.2–97.9 U/mL |
710 (32.4) |
202 (27.5) |
4,401 (30.1) |
|
|
≥ 98.9 U/mL |
1,192 (54.4) |
447 (60.9) |
7,982 (54.5) |
|
|
Unknown |
289 (13.2) |
84 (11.4) |
2,244 (15.3) |
|

Outcomes based on the five possible surgical approaches are shown in
Table 3. The average number of regional lymph nodes examined was significantly higher in the R-PD group (23.2 ± 12.2), even after conversion to open (22.4 ± 13.2), than that in the L-PD (19.1 ± 9.6), L-CO (18.8 ± 9.7), or O-PD (17.6 ± 9.5) group (all
p < 0.001). Margin positivity was higher in the converted cohort (R-CO = 36.6%, L-CO = 25.4%) than in other groups (
p = 0.017). The LOS (days) was shorter in the MI-PD group (L-PD 10.4 ± 8.6, R-PD 10.6 ± 8.8) than in the converted group (R-CO 10.7 ± 6.4, L-CO 11.2 ± 9.0) and the O-PD group (11.5 ± 8.9) (all
p < 0.001). There was no statistically significant difference in 30-day readmission (
p = 0.219), 30-day mortality (
p = 0.628), or 90-day mortality (
p = 0.520) among groups.
Table 3
Outcomes of patients undergoing pancreaticoduodenectomy
|
R-PD (%) |
R-CO (%) |
L-PD (%) |
L-CO (%) |
O-PD (%) |
p-value |
|
Number (%) |
405 (2.3) |
71 (0.4) |
1,786 (10.2) |
663 (3.8) |
14,645 (83.4) |
- |
|
No. of nodes |
23.2 ± 12.2 |
22.4 ± 13.2 |
19.1 ± 9.6 |
18.8 ± 9.7 |
17.6 ± 9.5 |
< 0.001*
|
|
Surgical margins |
|
|
|
|
|
0.017*
|
|
Negative |
313 (77.3) |
45 (63.4) |
1,385 (78.7) |
490 (74.6) |
11,237 (77.4) |
|
|
Positive |
92 (22.7) |
26 (36.6) |
375 (21.3) |
167 (25.4) |
3,273 (22.6) |
|
|
LOS (days) |
10.6 ± 8.8 |
10.7 ± 6.4 |
10.4 ± 8.6 |
11.2 ± 9.0 |
11.5 ± 8.9 |
< 0.001*
|
|
30-day readmission |
|
|
|
|
|
0.219 |
|
Yes |
38 (9.4) |
9 (12.7) |
148 (8.3) |
73 (11.0) |
1,416 (9.7) |
|
|
No |
367 (90.6) |
62 (87.3) |
1,627 (91.7) |
589 (89.0) |
13,188 (90.3) |
|
|
30-day mortality |
|
|
|
|
|
0.628 |
|
Yes |
11 (3.6) |
24 (6.0) |
40 (2.8) |
20 (3.6) |
405 (3.3) |
|
|
No |
291 (96.4) |
47 (94.0) |
1,398 (97.2) |
533 (96.4) |
12,024 (96.7) |
|
|
90-day mortality |
|
|
|
|
|
0.520 |
|
Yes |
17 (5.7) |
24 (6.0) |
88 (6.2) |
45 (8.2) |
842 (6.8) |
|
|
No |
284 (94.4) |
47 (94.0) |
1,338 (93.8) |
505 (91.8) |
11,508 (93.2) |
|

Additional non-surgical cancer therapies received by patients are demonstrated in
Table 4. The CO-PD group was most likely to receive neoadjuvant systemic therapy (15.7%) and least likely to receive adjuvant systemic therapy (47.7%) compared to other groups. Those in MI-PD and CO-PD groups were significantly more likely to receive at least two courses of systemic therapy treatments before surgery and at least two more after surgery (
p < 0.001). The average time from diagnosis to surgery was higher in the CO-PD group (59.5 ± 77.2 days) and the MI-PD group (55 ± 76.9 days) than in the O-PD group (51.1 ± 72.9 days) (
p = 0.001). The average time from diagnosis to systemic therapy was lower in the MI-PD group (63.6 ± 41.6 days) and the CO-PD group (64.6 ± 42.1 days) than in the O-PD group (66.4 ± 41.3 days) (
p = 0.040). Radiation therapy status (
p = 0.710), days from diagnosis to radiation (
p = 0.139), and total dose of radiation (
p = 0.404) did not show a statistically significant difference among groups.
Table 4
|
MI-PD (%) |
CO-PD (%) |
O-PD (%) |
p-value |
|
Systemic therapy sequence |
|
|
|
< 0.001*
|
|
None |
575 (26.4) |
197 (26.9) |
4,111 (28.3) |
|
|
Neoadjuvant |
313 (14.4) |
115 (15.7) |
1,934 (13.3) |
|
|
Adjuvant |
1,080 (49.6) |
349 (47.7) |
7,440 (51.2) |
|
|
≥ 2 neoadjuvant + ≥ 2 adjuvant |
210 (9.6) |
71 (9.7) |
1,036 (7.1) |
|
|
Days to surgery |
55.0 ± 76.9 |
59.5 ± 77.2 |
51.1 ± 72.9 |
0.001*
|
|
Days to systemic |
63.6 ± 41.6 |
64.6 ± 42.1 |
66.4 ± 41.3 |
0.040*
|
|
Days to radiation |
137.1 ± 80.7 |
136.6 ± 91.9 |
131.0 ± 81.4 |
0.139 |
|
Radiation therapy status |
|
|
|
0.710 |
|
Neoadjuvant |
202 (9.2) |
75 (10.2) |
1,369 (9.4) |
|
|
Adjuvant |
1,989 (90.8) |
659 (89.8) |
13,269 (90.7) |
|
|
Total radiation (Gy) |
5,974.0 ± 21,112.9 |
7,217.9 ± 23,602.4 |
6,333.3 ± 21,711.8 |
0.404 |

KM curves with log-rank tests were used to assess whether there were statistically significant differences in OS between surgical approaches. As shown in
Fig. 1, among patients who lived beyond 30 days after surgery, there were statistically significant differences among the three surgical approach groups (
p = 0.006). MI-PD displayed the highest median OS of 23.5 months (R-PD = 23.1 months, L-PD = 23.5 months), higher than 22.0 months in the O-PD group and 21.7 months in the CO-PD group (
Table 5). There was no statistically significant difference in OS or long-term survival between the two conversion types (
p = 0.247).
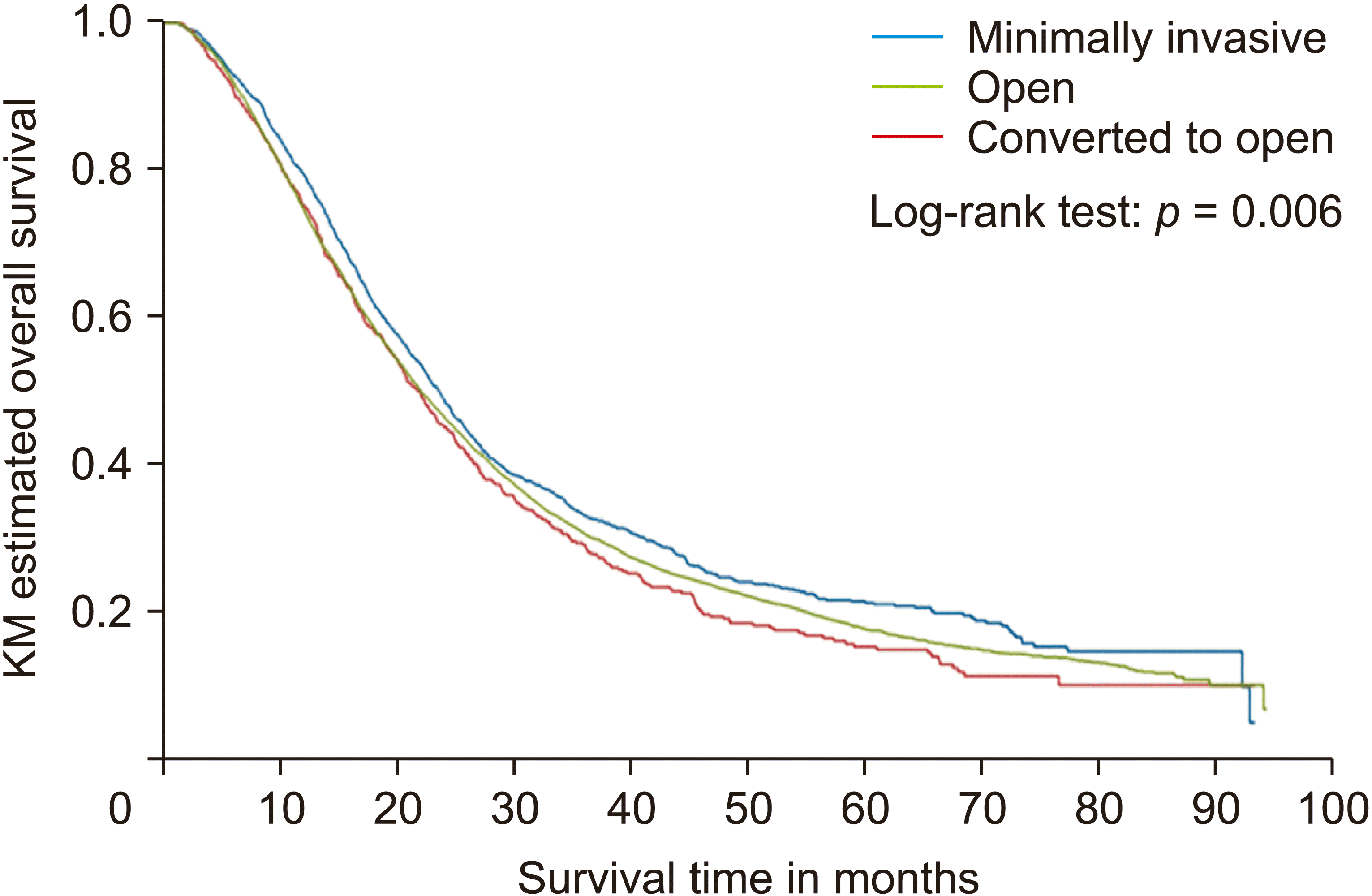 | Fig. 1Overall Survival by pancreaticoduodenectomy surgical approach. KM, Kaplan-Meier. 
|
Table 5
Median overall survival by surgical approach
|
Surgical approach |
Number |
Median overall survival (mon) (95% CI)a)
|
|
Open |
12,023 |
22.0 (21.6, 22.5) |
|
Minimally invasive |
1,688 |
23.5 (22.4, 24.6) |
|
Robotic |
291 |
23.1 (20.3, 26.2) |
|
Laparoscopic |
1,397 |
23.5 (22.4, 24.6) |
|
Converted-to-open |
580 |
21.7 (19.9, 23.4) |
|
Robotic-to-open |
47 |
20.5 (16.3, 24.9) |
|
Laparoscopic-to-open |
533 |
22.0 (19.4, 23.9) |

Results of binary survival outcomes assessed are shown in
Table 6. After adjusting for patient, clinical, tumor characteristics, and facility factors, there was no statistical difference in 30-day or 90-day mortality between groups. Both MI-PD (odds ratio [OR] = 1.40,
p < 0.001) and CO-PD (OR = 1.24,
p = 0.020) were significantly associated with an increased likelihood of long-term survival compared to the open approach, with MI-PD having a stronger association with long-term survival than CO-PD.
Table 6
Logistic regression examining the association of mortality and survival between surgical approaches
|
Outcome |
OR (95% CI) |
p-value |
AOR (95% CI)a)
|
p-value a)
|
|
30-day mortality |
|
|
|
|
|
Open |
Ref |
Ref |
Ref |
Ref |
|
Minimally-invasive |
0.90 (0.67, 1.20) |
0.469 |
1.00 (0.73, 1.36) |
0.985 |
|
Converted to open |
1.18 (0.77, 1.81) |
0.455 |
1.07 (0.68, 1.70) |
0.769 |
|
90-day mortality |
|
|
|
|
|
Open |
Ref |
Ref |
Ref |
Ref |
|
Minimally-invasive |
0.88 (0.72, 1.09) |
0.252 |
0.99 (0.79, 1.22) |
0.897 |
|
Converted to open |
1.19 (0.88, 1.61) |
0.264 |
1.09 (0.79, 1.51) |
0.593 |
|
Long-term survival |
|
|
|
|
|
Open |
Ref |
Ref |
Ref |
Ref |
|
Minimally-invasive |
1.42 (1.28, 1.57) |
< 0.001*
|
1.40 (1.25, 1.56) |
< 0.001*
|
|
Converted to open |
1.22 (1.02, 1.46) |
0.028*
|
1.24 (1.04, 1.49) |
0.020*
|

A statistically significant difference was also noted in median OS among the four quartiles of case-volume per year as indicated in KM curves with log-rank tests (
Fig. 2), with a trend of increasing median OS as case-volume per year increased from low-volume to very-high volume (
Table 7).
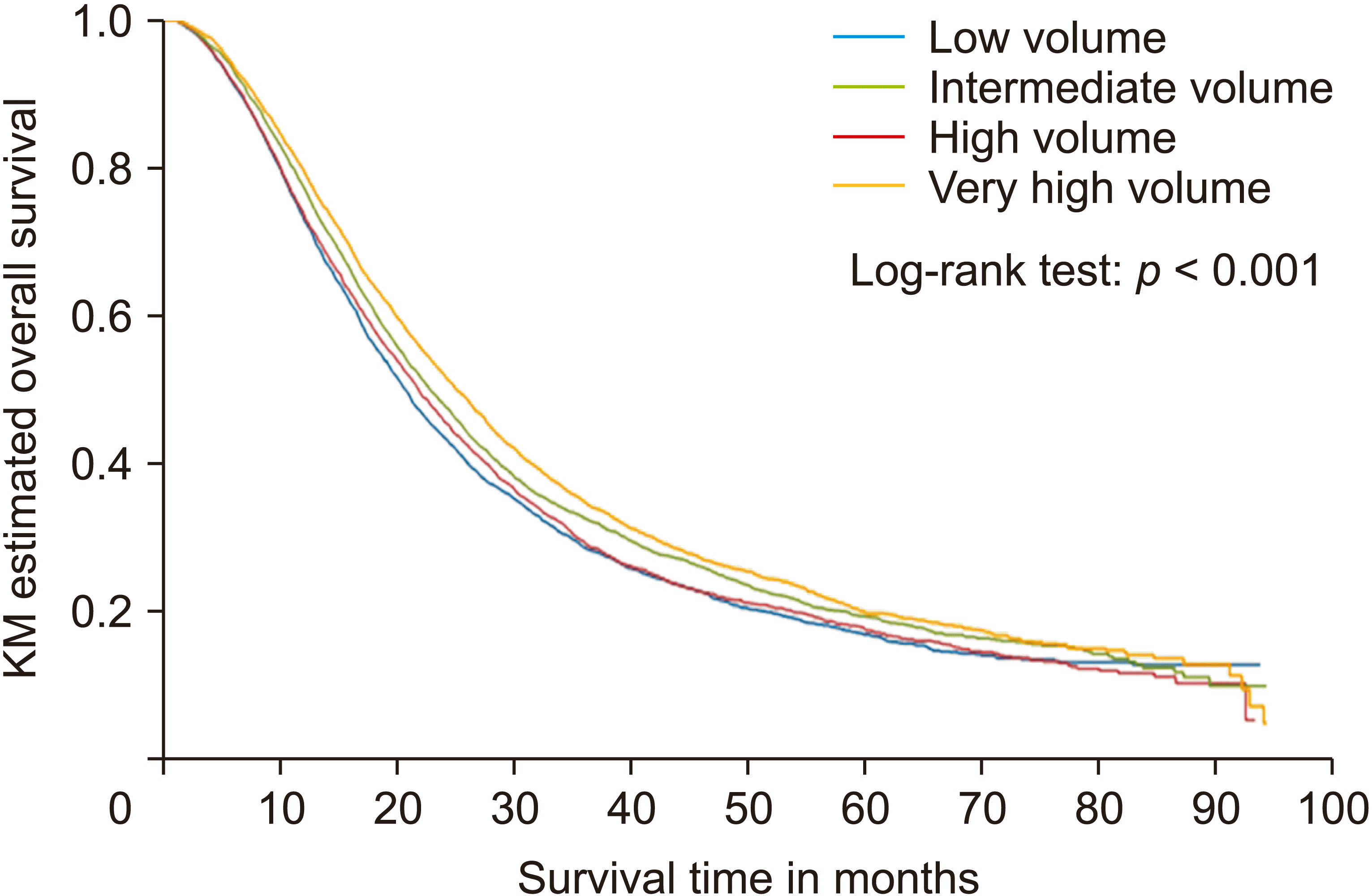 | Fig. 2Overall survival by case volume per year quartiles. KM, Kaplan-Meier. 
|
Table 7
Median overall survival by case-volume per year quartiles
|
Case volume |
Number |
Median overall survival (mon) (95% CI) |
|
Low |
4,280 |
20.7 (20.0, 21.3) |
|
Intermediate |
4,058 |
21.8 (21.1, 22.6) |
|
High |
4,323 |
22.8 (22.0, 23.7) |
|
Very high |
4,388 |
25.9 (24.2, 26.1) |

Go to :

DISCUSSION
Pancreaticoduodenectomy remains the only option for curing pancreatic head malignancies [
8,
9]. Since it was first reported by Kausch [
10] and Whipple et al. [
11] in 1935, operative technique, surgical technology, and perioperative care have improved. The high mortality rate has decreased from 30% in early years to 3%–5% in recent studies [
10-
12]. Despite this, O-PD still shows a significant morbidity [
12]. Minimally invasive techniques have been developed to minimize morbidity of the operation. Unfortunately, MI-PD, whether it be laparoscopic or robotic, is a technically demanding procedure with a steep learning curve [
4-
6]. Nonetheless, available data suggest that MI-PD is safe. In addition, MI-PD can improve short-term outcomes with at least equivalent oncologic outcomes in terms of lymph node yield as well as surgical margins. One systematic review of 12 studies comparing MI-PD to O-PD has reported that MI-PD is associated with reduced blood loss, shortened LOS, and comparable complication rates to O-PD [
13]. A review of the 2010–2016 NCDB comparing long-term oncologic outcomes of O-PD versus R-PD has found an equivalent, non-statistically significant difference in survival between R-PD (22 months) and O-PD (21.8 months) groups [
14]. However, R-PD was associated with a 20% decrease in risk of prolonged LOS compared to open procedures [
14].
Despite data demonstrating favorable outcomes with MI-PD, many surgeons remain hesitant to its adoption [
15]. One concern is the potential negative impact of conversion to O-PD as it pertains to both perioperative and oncologic outcomes. This is a concern not only fueled by the inherent complexity of the procedure and the known steep learning curve, but also fueled by the belief that conversion is considered a “surgical failure” (thus having poorer outcomes), at least partially. Although the impact of conversion at the time of MI-PD has not been quantified yet, apprehension is warranted given that prior studies have confirmed adverse impact of conversion at the time of attempted minimally invasive distal pancreatectomy (MI-DP). Nassour et al. [
16] have published a retrospective review of 2,926 distal pancreatectomies (open = 48.8%, minimally invasive = 42.8%, converted = 7.9%) using the American College of Surgeons National Surgical Quality Improvement Program database. The overall conversion rate was 15.3% (17.3% for the laparoscopic cohort and 8.5% for the robotic cohort). In their series, conversion was associated with a higher overall complication rate than successfully completed MI-DP and open distal pancreatectomy (O-DP) after adjusting for baseline patient characteristics. Although unadjusted, conversion was furthermore associated with a higher 30-day mortality. However, the potential influence of conversion on oncologic outcomes was not addressed [
16].
The impact of conversion on oncologic outcomes has been examined in the colorectal literature. Yerokun et al. [
17] have queried the 2010–2012 NCDB and identified 104,400 patients (minimally invasive = 38.6%, open = 55.5%, converted = 5.9%) who have undergone colectomy for a non-metastatic colon cancer. After adjusting for patient characteristics, no difference was noted in margin positivity rate between the converted cohort and the open cohort [
17]. However, the converted group had a slightly improved lymph node yield and shorter LOS. Conversion was also associated with a significantly decreased odds of 30-day mortality when compared to open colectomy [
17]. The authors’ conclusion was that conversion did not compromise oncologic outcomes and continued to maintain improved short-term outcomes.
Our analysis of over 17,000 patients undergoing pancreaticoduodenectomy for pancreatic cancer demonstrated that conversion at the time of attempted MI-PD did not adversely impact short-term or oncologic outcomes when compared to the current standard open approach. Based on the logistic regression, the likelihood of 30-day and 90-day mortality (i.e., short-term mortality) did not significantly differ among the three groups. While the NCDB neither gathers nor reports specific postoperative complication data, no statistical difference was noted in 30-day readmission which could be interpreted as a surrogate for perioperative complications. As it relates to median OS, CO-PD had the lowest OS among the three groups (21.7 months vs. 22.0 months and 23.5 months for O-PD and MI-PD, respectively). However, in terms of long-term survival (i.e., having survived for five years or longer), after adjusting for patient, clinical, tumor characteristics, and facility factors, both MI-PD (OR = 1.40,
p < 0.001) and CO-PD (OR = 1.24,
p = 0.030) were associated with an increased likelihood of long-term survival compared to O-PD. Although this survival advantage was conflicting with the study of Nassour et al. [
18] who reviewed the same 2010–2016 NCDB database and revealed a similar OS for R-PD and O-PD groups, our observed increased likelihood of long-term survival in the MI-PD and CO-PD group might be attributed to our larger sample size of patients given that we did not exclude L-PD from the analysis.
Although not the primary intent of the study, our data showed that successful completion of MI-PD seemed to offer improved outcomes when compared to O-PD. More importantly, it was interesting to note that not all benefits attained from a minimally invasive approach were lost if conversion to open was required. Lymph node yield was still significantly greater in the CO-PD group (R-CO 22.4 ± 13.2, L-CO 18.8 ± 9.7) than in the O-PD group (17.6 ± 9.5) (p < 0.001). When compared to patients undergoing O-PD, CO-PD was still associated with a shorter postoperative LOS (p < 0.001). Some enduring benefits noted in the CO-PD group were likely related to the time at which the case was converted, the reason for which the case required conversion, and the initial approach taken (robotic versus laparoscopic). For example, while the R-CO group had a higher lymph node yield than the L-CO group (22.4 ± 13.2 vs. 18.8 ± 9.7), the L-CO experienced a lower margin positivity rate than its R-CO counterpart (21.3% versus 36.6%).
Although MI-PD and CO-PD groups had similar pathologic stage (p = 0.277), tumor size (p = 0.652), lymphovascular invasion (p = 0.036), and regional node positivity (p = 0.640) suggesting a similar preoperative selection criterion, the CO-PD experienced a higher surgical margin positivity rate (CO-PD = 36.1%, MI-PD = 27.5%, O-PD = 22.6%). The reasoning for this finding is likely multifactorial. Despite similar clinical characteristics, the CO-PD group was significantly more likely to have received neo-adjuvant chemotherapy (p ≤ 0.001). Although not statistically significant, the CO-PD group was also more likely to have received neoadjuvant radiation therapy. These two findings suggest a higher preoperative concern for locally advanced disease in this cohort of patients. Although the CO-PD group had a higher margin positivity rate (36.1%), the R-CO (36.6%) margin positivity rate was statistically significantly higher than the L-CO (25.4%) margin positivity rate (p = 0.001). These variances suggest differences in the time or reason for conversion or advantages/limitations of a specific approach. Was the conversion performed before or upon completion of lymphadenectomy? Did the conversion occur during or after resection of pancreatic head? Was the case converted during the reconstruction? Although the NCDB does not document the reason for conversion, the aforementioned findings imply that the timing and reason for conversion as well as the minimally invasive approach utilized can affect outcomes following conversion and that not all conversions are equal.
A statistically significant difference was noted in median OS among the four quartiles of case-volume per year as indicated in KM curves with log-tank tests. An increasing trend existed in the median OS as case-volume per year increased from low-volume to very-high volume. This can be attributed to increased experience and expertise at high case-volume facilities such as academic medical centers. These findings are not surprising since available literature has revealed that patients receiving surgery at high-volume centers (at least 20 per year) experience improved perioperative outcomes [
19].
While we cannot determine why some patients were offered a minimally invasive approach, data suggested that such decision might have been patient driven. Data showed that many patients might have preferentially sought out surgeons and institutions that could offer MI-PD. Patients who were offered a minimally invasive approach, whether or not successfully completed, were significantly more likely to have traveled further for their care (MI-PD = 82.8 ± 252.9, CO-PD 71.4 ± 210.2) than the O-PD group (57.4 ± 154.3) as measured by the NCDB Mean Crowfly data point (p < 0.001). Accordingly, the average time from diagnosis to surgery was higher in those who underwent a minimally invasive approach (MI-PD = 55 ± 76.9 days, CO-PD = 59.5 ± 77.2 days vs. O-PD = 51.1 ± 72.9 days; p = 0.001), a finding likely attributable to additional travel and schedule related delays. Interestingly, despite the prolonged time from diagnosis to surgery, a lower average time from diagnosis to systemic therapy was noted in the minimally invasive group (MI-PD = 63.6 ± 41.6 days, CO-PD = 64.6 ± 42.1 days vs. O-PD group 66.4 ± 41.3 days; p = 0.040). This finding is at least partially attributable to increased utilization of neoadjuvant chemotherapy noted in the minimally invasive group (14.4% in MI-PD, 15.7% in CO-PD vs. 13.3% in O-PD, p < 0.001). The lower average time from diagnosis to systemic therapy might also be reflective of expedited recovery noted in the minimally invasive group as measured by shortened LOS and its potential impact on earlier initiation of adjuvant chemotherapy.
Our study has several limitations inherent to its retrospective design. Despite our best effort to adjust for known clinical variables, there might be an inherent element of selection bias seen in all retrospective reviews. As it pertains to accurately assessing the impact of MI-PD on short-term outcomes, the NCDB unfortunately does not gather nor report data on specific short-terms complications. Therefore, we were limited in obtaining available surrogate metrics such as LOS, readmission, and 30-day mortality. That said, while our analysis of NCDB data suggests that even when requiring conversion MI-PD offers advantages over O-PD, a causal relationship cannot fully be made. Second, neither the experience of the physician performing the procedure nor the reason for conversion are known. The comfort level of each physician prior to performing MI-PD was also unknown. Despite these limitations, our study is the first to assess the impact of conversion on outcomes following attempted MI-PD. Results of this observational study may serve to complement or inform the development of future randomized controlled trials to determine best practices.
In conclusion, our analysis of patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma demonstrates that conversion at the time of attempted MI-PD does not adversely impact perioperative nor oncologic outcomes when compared to O-PD. Although MI-PD seems to be associated with improved short and long-term benefits when compared O-PD, only 16.7% of patients are offered a minimally invasive approach. That said, MI-PD should be considered safe, even in the setting of fear for conversion. It is possibly preferred in patients who do not have any contraindication to a minimally invasive approach. Despite the inherent complexity and hesitancy to adopt this procedure into common practice, surgeons should seek to familiarize themselves and possibly integrate this technique into their practice.
Go to :





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download