RESULTS
Fig. 1 summarizes the demographic distribution of the study population. For the 100 cases under consideration in this study, the mean age was 39.5 ± 11.6 years. More than half of the cases were present in the 21–40 yr age group (58.0%). The youngest case was 15 years old, while the oldest was 81 years old. The lowest mean age was among the cases of chronic cholecystitis 35.6 ± 11.0 yr, and was 49.5 ± 9.1 yr for metaplasia, 50.8 ± 14.6 yr for dysplasia, and 55.0 ± 16.9 yr for gall bladder carcinoma.
Fig. 1 shows that there was a higher number of female patients with female cases being 81.0%, with the female to male ratio being 4.1:1.
Table 1 describes the case distribution per number (solitary, multiple) and size of the stones. The majority of the cases had multiple stones (n = 69). The maximum number of patients (n = 52) had stone size in the range (11–20 mm).
Table 2 enumerates the histopathological diagnoses made on microscopy examination, and the age and sex distribution of the respective diagnoses. There were 5 cases (5.0%) of carcinoma gall bladder, of which 4 were female. The majority of cases of chronic cholecystitis were in the age group (21–40 yr) (n = 52). Among them, 38.7% cases (29/75 cases) of chronic cholecystitis were seen in the 21–30 yr age group. The mean age of chronic cholecystitis was 35.0 ± 11.6 yr. Twelve cases of metaplasia and 8 cases of dysplasia were observed. Age group comparison of metaplasia and carcinoma with respect to chronic cholecystitis showed significant
p-value (metaplasia, < 0.001; dysplasia, 0.15), indicating that there were statistically significant more cases of metaplasia and carcinoma among the older age groups of 41–60 and > 60 years. Among chronic cholecystitis, the maximum cases were of Rokitansky-Aschcoff sinus (
Fig. 2). There were 7 cases of intestinal metaplasia (
Fig. 3), 3 cases of pyloric metaplasia, and 2 cases of squamous metaplasia. Four cases had adenocarcinoma GB (
Fig. 4), while one was suffering from squamous cell carcinoma GB.
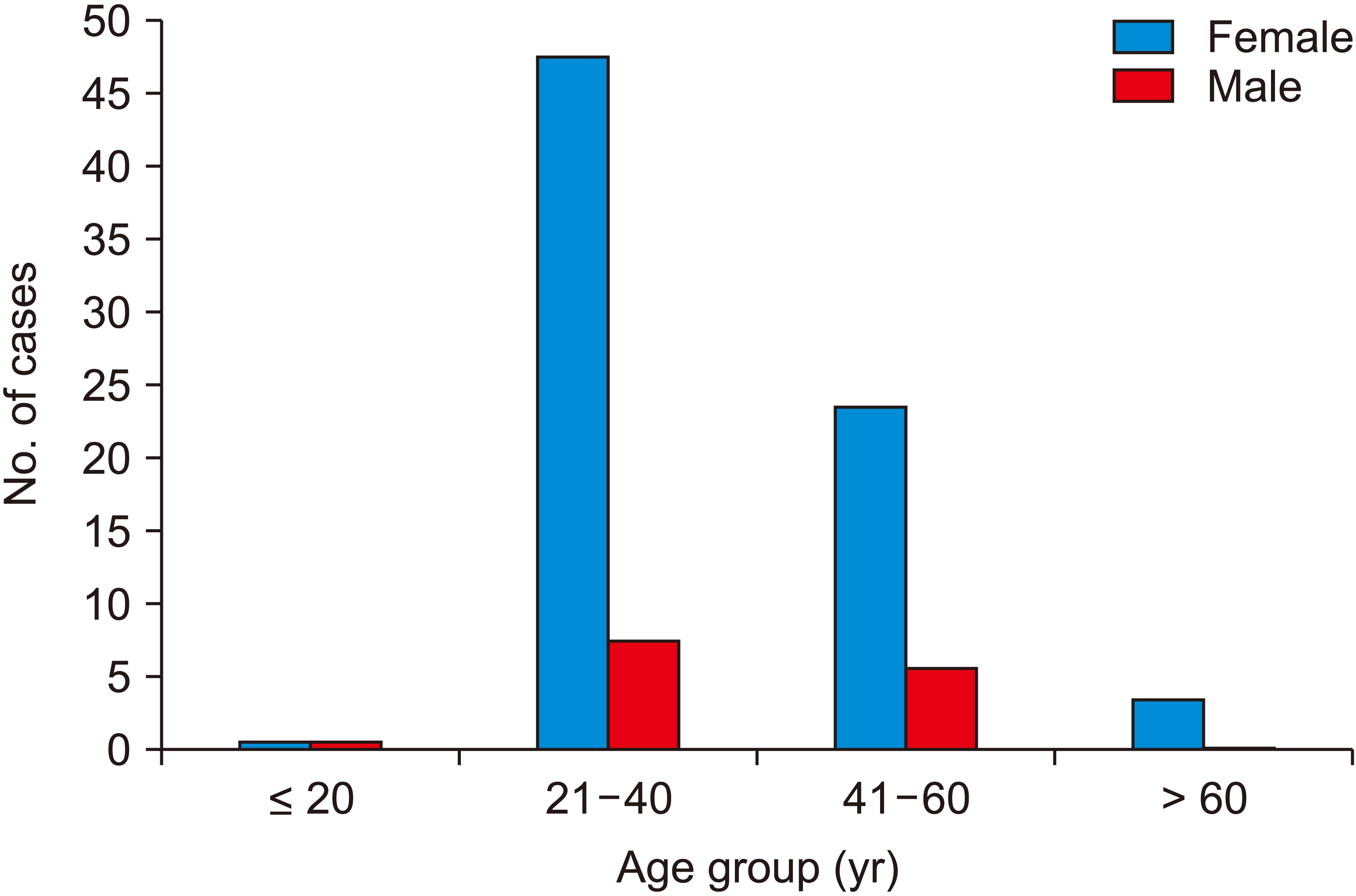 | Fig. 1Sex distribution of age groups (years). 
|
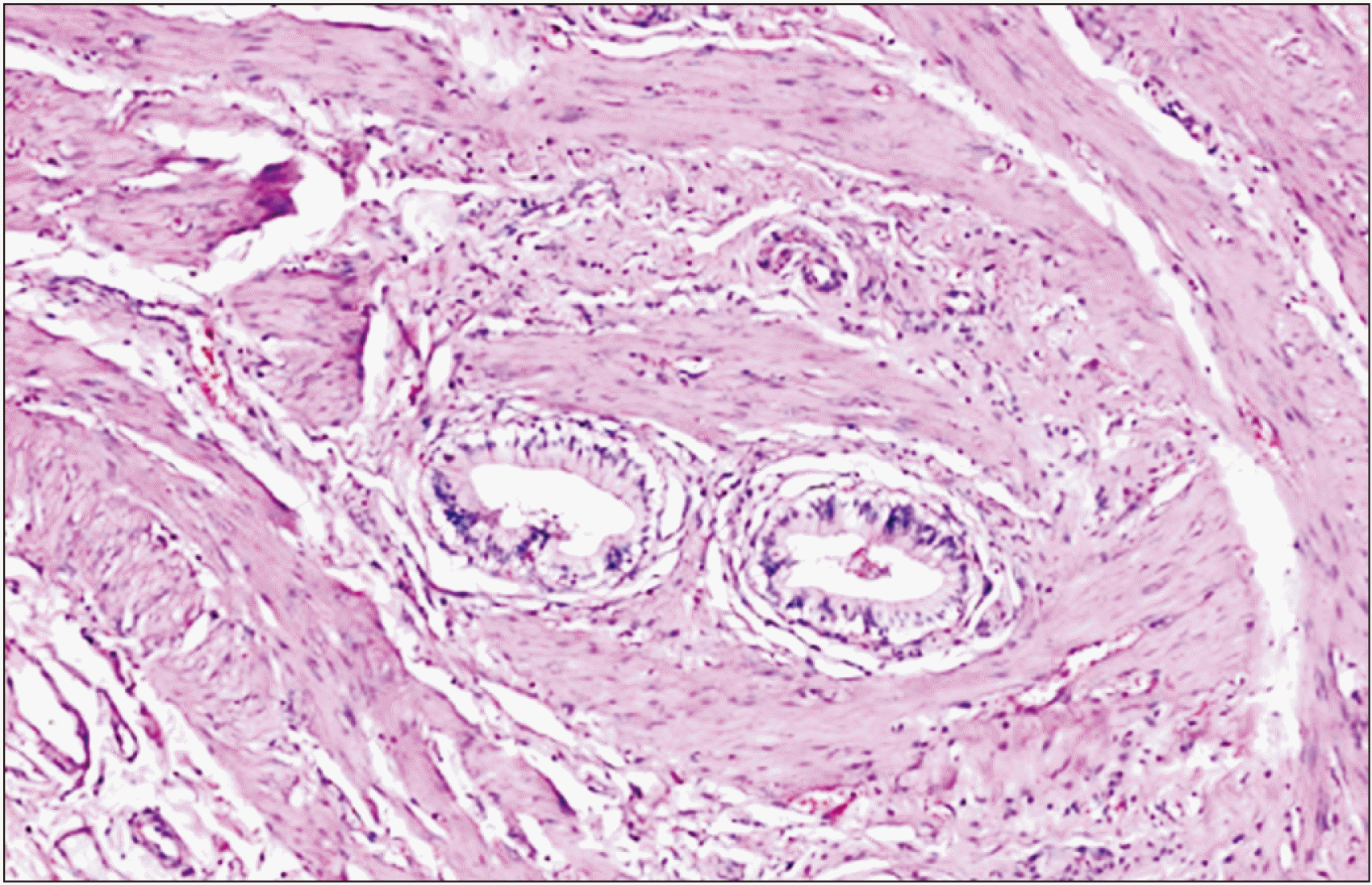 | Fig. 2Chronic cholecystitis with Rokitansky-Aschoff sinus (H&E, ×10). 
|
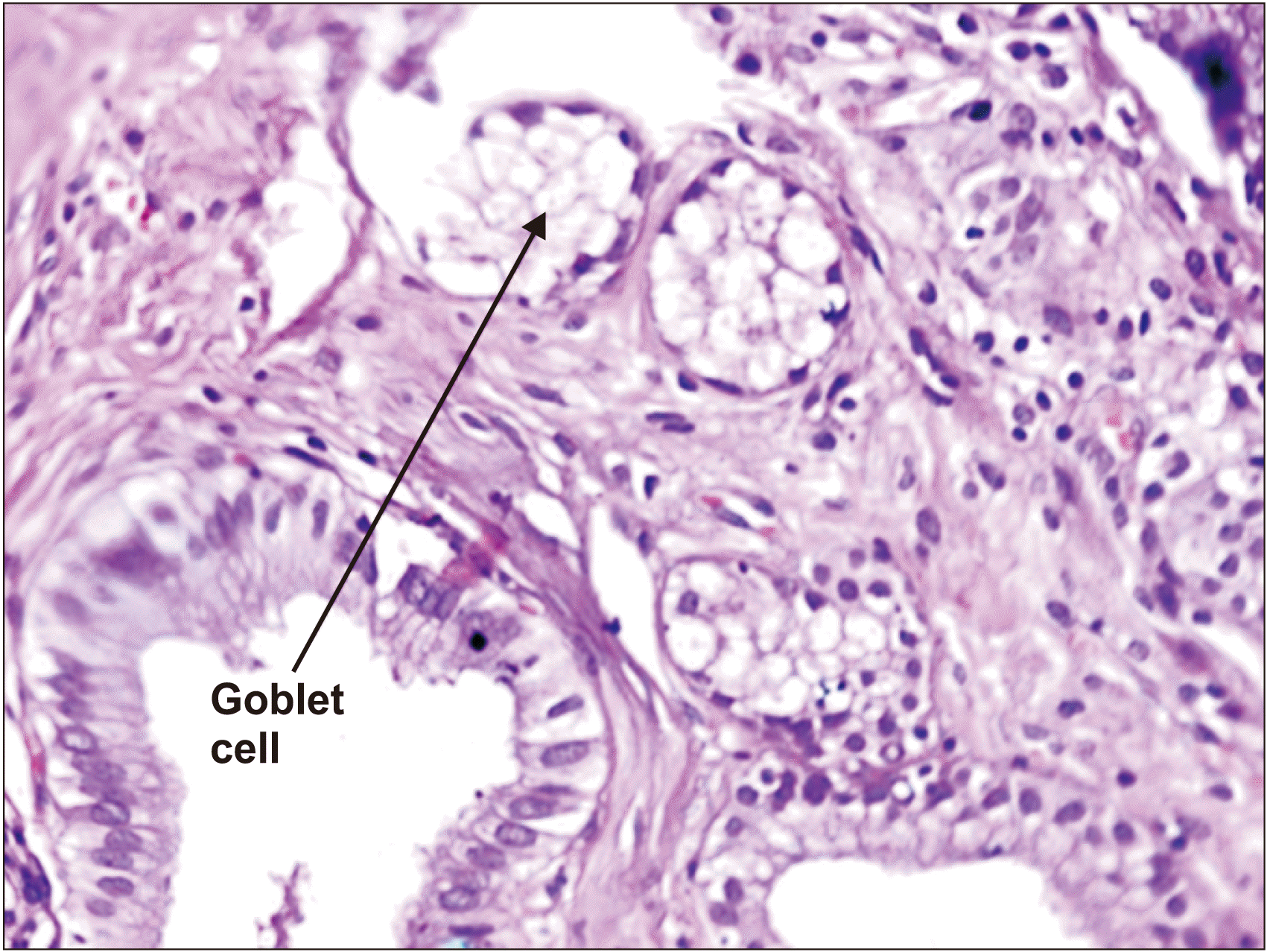 | Fig. 3Intestinal metaplasia, gallbladder (H&E, ×40). 
|
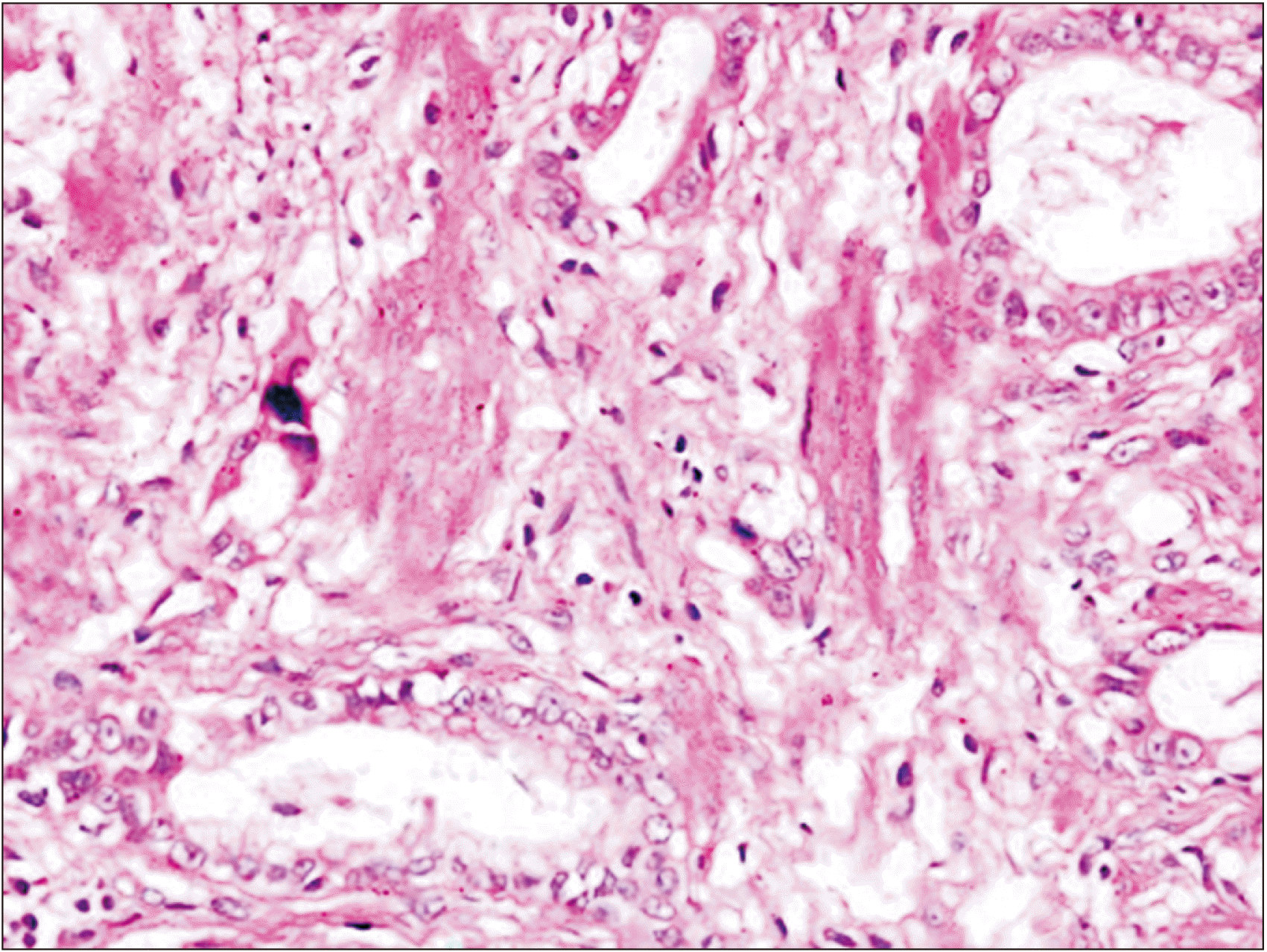 | Fig. 4Adenocarcinoma gallbladder (H&E, ×40). 
|
Table 1
Case distribution per number and size of gallstones
|
Stone |
Case (n) |
Percentage (%) |
|
Number |
|
|
|
Solitary |
31 |
31.0 |
|
Multiple |
69 |
69.0 |
|
Total |
100 |
100 |
|
Size (mm) |
|
|
|
< 6 |
2 |
2.0 |
|
6–10.9 |
26 |
26.0 |
|
11–20 |
52 |
52.0 |
|
> 20 |
20 |
20.0 |
|
Total |
100 |
100 |

Table 2
Sex and age group distribution of the respective histopathological diagnoses
|
Histopathological diagnosis (further classified as per sub types) |
Age group (yr) |
Sex |
Total |
p-value |
|
|
Female |
Male |
|
CC |
|
|
|
|
|
|
Non-specific CC-20 |
≤ 20 |
2 |
2 |
4 |
|
|
Dyst, calcification-6 |
21–40 |
45 |
7 |
52 |
|
|
RA sinus-24 |
41–60 |
15 |
2 |
17 |
|
|
RA sinus and cholesterolosis-10 |
> 60 |
2 |
0 |
2 |
|
|
Total |
|
64 |
11 |
75 |
|
|
Metaplasia |
|
|
|
|
< 0.001 |
|
Intestinal M”-7 |
21–40 |
1 |
1 |
2 |
|
|
Pyloric M”-3 |
41–60 |
4 |
4 |
8 |
|
|
Squamous M”-2 |
> 60 |
2 |
0 |
2 |
|
|
Total |
|
7 |
5 |
12 |
|
|
Dysplasia |
|
|
|
|
0.15 |
|
21–40 |
3 |
0 |
3 |
|
|
41–60 |
3 |
1 |
4 |
|
|
> 60 |
0 |
1 |
1 |
|
|
Total |
|
6 |
2 |
8 |
|
|
Carcinoma |
21–40 |
0 |
1 |
1 |
0.04 |
|
Adenocarcinoma gallbladder-4 |
41–60 |
3 |
0 |
3 |
|
|
Squamous cell carcinoma-1 |
> 60 |
1 |
0 |
1 |
|
|
Total |
|
4 |
1 |
5 |
|

The relation between histopathological diagnoses and the number of stones was studied (
Table 3). Among 69 cases of multiple stones, there was only 1 case (1.45%) of carcinoma GB, whereas among 31 cases of solitary stone, there were 4 such cases (12.90%). Among the multiple stone cases, there were 54 cases (78.26%) of chronic cholecystitis, and among the solitary stone cases, 21 cases (67.74%) of chronic cholecystitis. There was no concomitant case of GB polyp with cholelithiasis. Chi-square test was applied for the above comparison; however, the calculated
p-value was not found to be significant.
Table 3
Comparison of the respective histopathological diagnoses with respect to the number of gallstones (solitary/multiple)
|
Histopathological |
Multiple stones |
|
Solitary stone |
|
Total |
p-value |
|
|
|
|
Case (n) |
Percentage (%) |
Case (n) |
Percentage (%) |
n |
Percentage (%) |
|
Chronic cholecystitis |
54 |
78.26 |
|
21 |
67.74 |
|
75 |
75.00 |
|
|
Metaplasia |
9 |
13.04 |
|
3 |
9.68 |
|
12 |
12.00 |
|
|
Dysplasia |
5 |
7.25 |
|
3 |
9.68 |
|
8 |
8.00 |
|
|
Carcinoma |
1 |
1.45 |
|
4 |
12.90 |
|
5 |
5.00 |
|
|
Total |
69 |
100.00 |
|
31 |
100.00 |
|
100 |
100.00 |
0.09 |

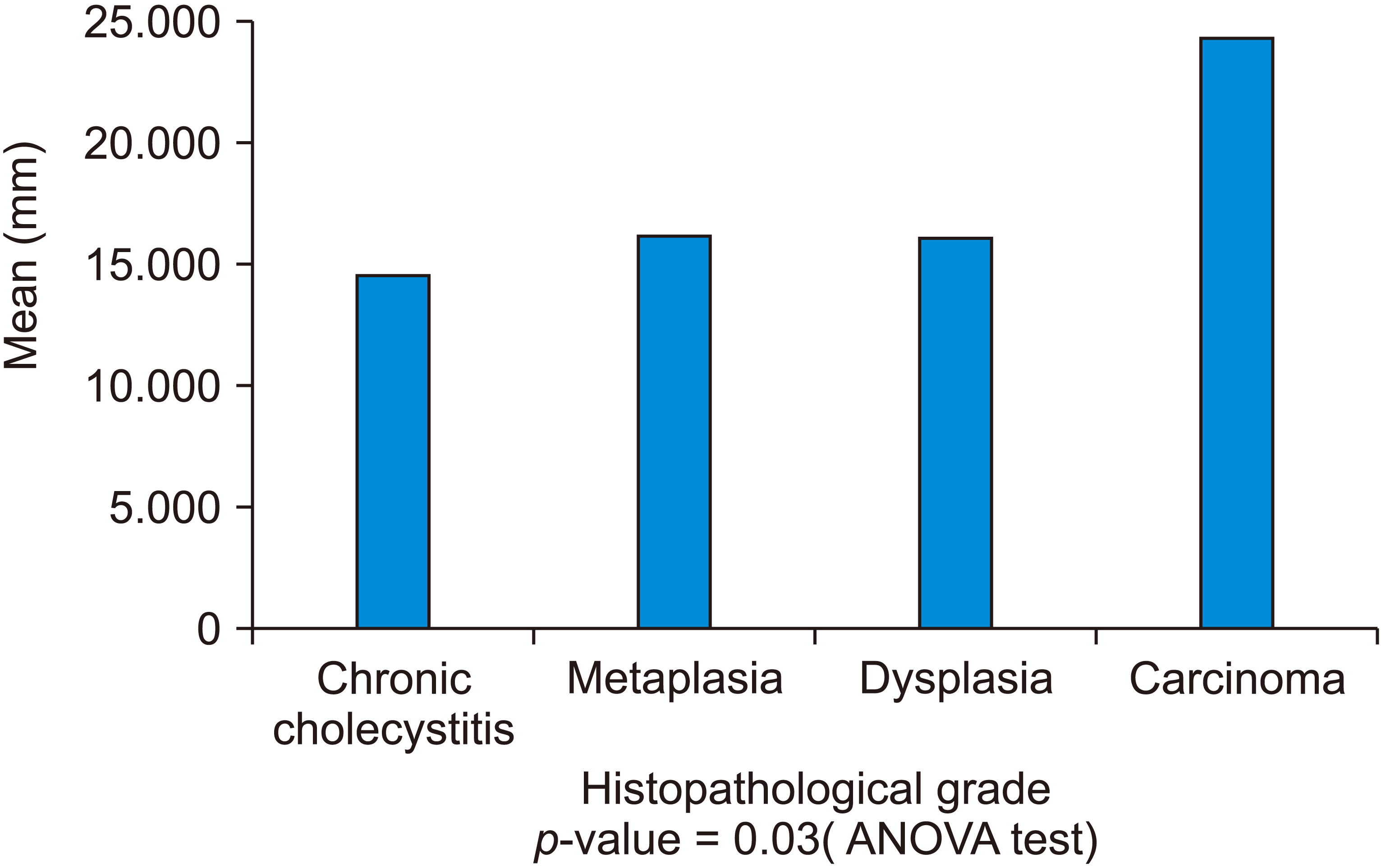
The average calculated diameters of the stone of respective histopathological diagnoses were compared amongst with each other (
Fig. 5). In the case of chronic cholecystitis, the average diameter was 13.3 ± 6.2 mm, whereas for GB carcinoma, it was observed to be 23.1 ± 3.2 mm. The
p-value calculated using ANOVA test was significant for the above comparison (
p = 0.03).
Table 4 demonstrates the relation between GB thickness and histopathological diagnoses. Among the 36 cases of increased wall thickness (> 3 mm), 24 cases (66.67%) were of chronic cholecystitis, 5 cases (13.89%) had gall bladder metaplasia, 4 cases (11.11%) had dysplasia, and 3 cases (8.33%) were diagnosed with GB carcinoma. In the case of gall bladder carcinoma, the average wall thickness was 3.7 mm (
Table 4). The
p-value for the comparison of average wall thickness among the respective histopathological diagnoses was not significant (ANOVA test). Cases of respective histopathological diagnoses were tabulated according to the first 50 percentile as they were arranged in increasing order of age (
Table 5). For the first 50th percentile, the majority of cases were suffering from chronic cholecystitis (94.0%). For the last 50th percentile, 22 cases (44.0%) were diagnosed with preneoplastic (metaplasia, 22.0%; dysplasia, 14.0%), and neoplastic lesion (8.0%).
p-value was calculated using chi-square test, and was found to be highly significant (< 0.001). The average stone diameter of the first and last 50 percentile cases was calculated, and a statistical comparison was made, using paired t-test. Significant
p-value was observed (
p = 0.02).
Table 6 describes the univariate and multivariate analyses for various variables associated with GB carcinoma. The 5 cases of GB carcinoma were compared with the other cases of cholelithiasis. For univariate analysis, age (odds ratio [OR], 6.882;
p = 0.043), gallstone number (OR, 9.1;
p = 0.050), gallstone size (OR, 17.111;
p = 0.013), and duration of symptom (OR, 34.125;
p = 0.001) were found to have significant
p-value, as well as higher OR. So increasing age, solitary stone, larger stone, and increased duration of symptom were found to be associated with GB carcinoma. Only GB wall thickness (OR, 7.515;
p = 0.076) was found to be a non-significant risk factor for GB carcinoma. In the multivariate analysis performed for the risk factors described above, non-significant
p-value was calculated for GB carcinoma risk factors. In the multivariate (conditional) analysis performed for the duration of symptoms, the
p-value was significant (
p = 0.008; OR, 21.118). The confidence interval in univariate, as well as multivariate analysis, was found to have a wide range for all the variables, as the sample size was small, and there was a very uneven distribution of cases in the two statistical groups (carcinoma, 5 cases; other cases of cholelithiasis, 95).
 | Fig. 5Comparison of respective histopathological diagnoses with respect to size of gallstones (solitary/multiple). 
|
Table 4
Comparison between different histopathological diagnoses according to the wall thickness
|
Histopathology |
Gall bladder thickness ≤ 3 mm |
|
Gall bladder thickness > 3 mm |
Wall thickness (mean ± SD, mm) |
p-value |
|
|
|
n |
Percentage (%) |
n |
Percentage (%) |
|
Chronic cholecystitis |
51 |
79.69 |
|
24 |
66.67 |
2.8 ± 1.4 |
|
|
Metaplasia |
7 |
10.94 |
|
5 |
13.89 |
3.2 ± 1.4 |
|
|
Dysplasia |
4 |
6.25 |
|
4 |
11.11 |
3.5 ± 1.7 |
|
|
Carcinoma |
2 |
3.13 |
|
3 |
8.33 |
3.7 ± 0.7 |
|
|
Total |
64 |
100.00 |
|
36 |
100.00 |
|
0.42 |

Table 5
Case distribution according to various histopathological diagnoses as per the first 50 percentile and last 50 age percentile, and comparison of stone size of the first 50 percentile and last 50 age percentile
|
Histopathological diagnosis (cases) |
First 50 percentile of cases as arranged in increasing order of age |
|
Last 50 percentile of cases as arranged in increasing order of age |
p-value |
|
|
|
No |
Percentage (%) |
No |
Percentage (%) |
|
Chronic cholecystitis |
47 |
94 |
|
28 |
56 |
|
|
Preneoplastic (metaplasia and dysplasia) and neoplastic (carcinoma) cases |
3 |
6 |
|
22 |
44 |
|
|
Total |
50 |
100 |
|
50 |
100 |
< 0.001a)
|
|
Stone size (mean ± SD, mm) |
12.90 ± 5.52 |
15.50 ± 6.18 |
0.02b)
|

Table 6
Univariate and multivariate analysis of various variables associated with gallbladder carcinoma
|
Univariate |
|
Multivariate |
|
Multivariate (conditional) |
|
|
|
|
OR |
95% CI |
p-value |
OR |
95% CI |
p-value |
OR |
95% CI |
p-value |
|
Age (≤ 50 yr, > 50 yr) |
6.882 |
1.067–44.411 |
0.043 |
|
12.591 |
0.660–240.053 |
0.092 |
|
|
|
|
|
Wall thickness (≤ 3 mm, > 3 mm) |
7.515 |
0.807–70.004 |
0.076 |
|
1.159 |
0.056–24.213 |
0.924 |
|
|
|
|
|
Stone number (solitary, multiple) |
9.1 |
0.012–1.026 |
0.050 |
|
0.086 |
0.004–1.954 |
0.124 |
|
|
|
|
|
Stone size (≤ 20 mm, > 20 mm) |
17.111 |
1.802–162.440 |
0.013 |
|
4.761 |
0.337–67.221 |
0.248 |
|
|
|
|
|
Duration of symptoms (≤ 10 weeks, > 10 weeks) |
34.125 |
4.392–265.163 |
0.001 |
|
25.293 |
0.924–692.324 |
0.056 |
|
21.118 |
2.235–199.569 |
0.008a)
|

Go to :

DISCUSSION
Gallstone disease remains one of the major causes of abdominal morbidity and mortality globally. The prevalence rates are higher in North India than in South India. The old aphorism by William Mayo, “There is no innocent gallstone,” is a reflection on the fact that cholelithiasis generally causes inflammation in the gall bladder mucosa, which is associated with a series of histopathological changes that could be a precursor to cancer. These changes could be chronic cholecystitis, metaplasia, dysplasia, or carcinoma.
Maximum crowding of the cases was seen in the age group 20–40 yr (58%), and the incidence dropped in older age groups, with only 6% cases in the > 60 years age group. The calculated mean age was 39.5 ± 13.5 yr. Narang et al. [
2] demonstrated similar results with maximum cases (58%) in the 30–40 yr age group. Bansal et al. [
3] and Tyagi et al. [
4] reported the mean age of 43.6 and 42.5 yr, respectively, which was slightly older than the mean of our study. Childhood obesity due to high calorie diet (fried fast foods) and low fibre diet, in addition to sedentary lifestyle, might contribute toward the increasing incidence in the younger age group [
5].
In the present study, more than 2/3rds (68%) of the chronic cholecystitis cases (52/75) were present in the 21–40 yr age group. Among them, 39% cases (29/75 cases) of chronic chloecystitis were seen in the 21–30 yr age group. The mean age of chronic cholecystitis was 35 ± 11.6 yr. Shafique et al. [
6] observed similar results with 48% cases under the age of 30 yr. These findings were in contrast to Rakesh and Rajendra [
7], which observed 58% cases in the 40–60 yr group, and only 24% cases in the 20–40 yr age group. Khan et al. [
8] concluded that the use of oral contraceptive pills promoted lithogenicity, principally in metabolically susceptible younger age women. Diabetes mellitus has generally been linked to cholelithiasis and biliary tract inflammation. The underlying mechanisms may include fasting hyperinsulinemia [
9], and diabetic neurogenic GB [
10]. Shafique et al. [
6] showed high body mass index to be strongly associated with cholelithiasis. It can be summarised that the increased availability of OCP, rising cases of diabetes mellitus in the young population, and changing lifestyle and diet habits, with all 3 in conjunction, may be contributing to the increasing incidence of cholelithiasis in the young population.
The average age of GB cancer patients was 55 ± 16 yr, much older than the overall average age of 39.5 yr. Univariate analysis performed for age comparison among GB cancer and other cases of cholelithiasis found the
p-value (< 0.001) to be significant, signifying age to be a risk factor for GB carcinoma. Gupta et al. [
11] noted that the average age in gall bladder cancer was 51 ± 11 yr while Rathore et al. [
12] found the average age to be 52.4 yr. The mean age of presentation of GB carcinoma in India is younger than the average age in the USA and western European countries [
13]. This is possibly due to the presence of additional multiple risk factors, which increase the probability [
14].
In our study, there were a higher number of female patients with female cases being 81.0%, with the female to-male ratio being 4.1:1. The observed ratio of gender distribution was in accordance with Shafique et al. [
6], which had F:M ratio of (4.6:1). Women with more pregnancies and longer length of fertility periods are more likely to have gall stones, than those who remain nulliparous [
15]. Estrogen increases biliary cholesterol, causing supersaturation of the bile. Hence, hormone replacement therapy and oral contraceptives have also been described as being associated with an increased risk for gallstone disease [
16]. In the current study, among 5 cases of GB cancer, four cases were female, and only one case was male. Among the 81 female cases of cholelithiasis, 4 had carcinoma whereas, among 19 male cases of cholelithiasis, one had carcinoma, cancer incidence being 5% in both males and females. The age-standardized incidence rate of GB cancer for women, at 2.4 (per 100,000), is slightly higher than that for men, at 2.2 [
17]. The retrospective case series study by Konstantinidis et al. [
18] demonstrated (2–6) times risk of GB cancer in women. The role of female sex hormones is strengthened through GB cancer being associated with a high parity and greater number of pregnancies [
19]. The co-expression of estrogen and progesterone receptors is increased in females with GB cancer, as compared with males, signifying the role that these hormones play in the etiopathogenesis of GB cancer in females [
19].
In the present study, the majority of cases (69%) had multiple stones. Goyal et al. [
20] showed similar result, with multiple stones being 69.3%, and solitary stones being 30.6, while Nair et al. [
21] showed marginally different results, with multiple stones being 66%, and solitary stones 34%.
With respect to measured gallstone size, the maximum cases (47%) had stone size in the range 11–20 mm, and 25% cases were more than 25 mm in size. The above finding was in contrast to Alshoabi [
22], which observed maximum cases in the 5–10 mm range, and only 14.0% of cases were more than 20 mm in size.
In the present study, as per the histopathological diagnoses, 75% cases were of chronic cholecystitis, 12% had metaplasia, 8% were of dysplasia, and 5% cases had carcinoma. In a marginal variance to our findings, Goyal et al. [
20] emonstrated 63% cases of chronic cholecystitis, 19% cases of metaplasia, 4 % cases of dysplasia and 1% cases of carcinoma GB. Mathur et al. [
23] observed 60% cases of chronic cholecystitis, 8% cases of hyperplasia, and 2% cases of carcinoma. The probable causes for the increased incidence of gall bladder carcinoma are discussed later in the study.
Among the twelve cases (12.0%) of metaplasia, the majority had intestinal metaplasia (7%). Three cases of metaplasia had co-existing dysplasia. In contrast to our study, Khanna et al. [
24] observed pyloric metaplasia in 15% cases, and intestinal metaplasia in 16% cases. Five cases of metaplasia had increased wall thickness, and the average wall thickness was 3.2 ± 1.4 mm. Seretis et al. [
25] demonstrated that the increased incidence GB metaplasia was associated with increased wall thickness. The current study showed a rate of carcinoma of 5.0%, which is of great concern for healthcare providers, as well as the population itself. The GB cancer incidence rate of 11.8/100,000 in North India is the highest among Asian countries, and is comparable to the rates in high-incidence countries, such as Bolivia and Chile. The average age-adjusted rate among women increased from 6.2/100,000 in 2001–2004, to 10.4/100,000 in 2012–2014 [
26]. This data is from 30 population-based cancer registries from all over India, which were set up by the Indian Council of Medical Research (ICMR) [
27]. There are 90 cancer cases per lakh population in the state of Punjab, India, as compared to the average of 80 per lakh in the national average [
28]. High use of pesticides, presence of toxic material also in sub-soil water, leaching of uranium from rocks, and presence of thermal power plants based on coal are thought to the reasons for the high incidence of cancer in the state of Punjab, India. The work of Aggarwal et al. [
29] on the pattern of cancer incidence in the Malwa region of the Punjab, was published in March 2015, demonstrated breast cancer to be the leading cancer (35%) among all cancer cases, (most common), and 4% cases were of gall bladder cancer (fifth highest cases), whereas in a recent study published November 2021 by Budukh et al. [
30] on cancer cases reported in the Homi Bhaba cancer institute, Sangrur reported GB cancer being the 3rd most common cancer (7.9%). Here we can observe that the proportion of gall bladder cancer cases among all cancer cases significantly increased from 4% to almost 7.9% in the north Indian region of Punjab over a period of few years. Among five cases of carcinoma GB, the majority of the cases were of adenocarcinoma GB (4 cases). Hundal and Shaffer [
31] observed adenocarcinoma to be the most common histologic type, accounting for 98% of all GB tumours, two-thirds of which are moderately/poorly differentiated. Some 3 of 5 cases of ca. gall bladder displayed increased wall thickness (more than 3 mm), and the average wall thickness among carcinoma cases was 3.7 mm. But univariate and multivariate analysis did not show the wall thickness to be a significant variable associated with GB carcinoma. In high-risk areas for GB carcinoma (like north India), any GB wall thickness over 3 mm focally or diffusely with enhanced vascularity should alert the radiologist. Application of high-frequency probes, color Doppler, and the use of contrast agents has enhanced the discriminative ability of ultrasonography (USG) [
32]. In the cases of suspicious findings of USG, computerized tomography (CT) is an important tool to gauge early lesions, assess disease extensions into adjacent structures (especially the portal vein and hepatic artery), and the accurate stage of the disease, but the CT scan has limited use in detecting metastasis. Endoscopic ultrasound (EUS) has emerged as an important tool to diagnose early GB carcinoma, and stage of GB carcinoma more accurately than CT scan, and creates direct access to GB and lymph nodal tissue through fine needle aspiration [
33].
In the present study, for comparison among four individual histopathological diagnoses with the number of stones, no significant association was found between the number of stones and histopathological diagnoses. However, among 5 cases of gall bladder carcinoma, 4 had large solitary stones. Moreover, univariate analysis between GB carcinoma and all other cases of cholelithiasis for the number of gall stones, noted that the p value was significant. Roa et al. [
34] and Mathur et al. [
23], could not demonstrate any significant association between mucosal response and the number of stones. However, Diehl [
35] and Lowenfels et al. [
36] concluded that large solitary stones were found in the majority of the cases of GB carcinoma. Similarly, Narang et al. [
2] showed significant relation between the number of stones and malignant changes in gall bladder mucosa.
The correlation between the size of stone and histopathological changes in mucosa response done using ANOVA test was found to be statistically significant (
p < 0.03). This suggests that the size of gall stone in the case of carcinoma gall bladder was significantly more than in the case of metaplasia, dysplasia, and chronic cholecystitis. Moreover, univariate analysis demonstrated gallstone size to be an independent risk factor for GB carcinoma. Csendes et al. [
37] showed similar findings, where it was demonstrated that patients with carcinoma gall bladder had significantly larger stone, regardless of the number of stones present. Other studies also show positive association of size of gallstone with carcinoma GB [
36,
38,
39]. However, Moerman et al. [
40] did not establish any relationship between GB malignancy and stone size. Four out of five patients of carcinoma GB had large solitary stones. Verma et al. [
41] postulated that the large stones on attaining a considerable size tend to settle in the dependent part of the GB. With the passage of time, the stone increases in size, causing stretching of the wall of the GB around it, whereas the small floating stones may not remain in constant touch with the GB mucosa, thereby minimizing the severity of inflammation. Therefore, over the same period of time, large solitary stones due to constant irritation of the mucosa tend to cause more severe damage than multiple stones, which may justify the higher rate of carcinoma reported among solitary stones.
All 100 cases of the study were arranged in ascending order of age from youngest to oldest patient. Comparison of cases in the first 50 and last 50 age percentiles with respect to histopathological diagnoses yielded significant p-value (< 0.001). It was observed that the fraction of preneoplastic and neoplastic cases soared from 6.0% to 44.0% in the first to last 50 percentile cases. Average stone diameter of the stone size of the first 50 age percentile cases was found to be 12.9 mm, and for last 50 percentile cases, the average diameter was calculated to be 15.50 mm and the comparison yielded significant p-value (0.02).
So as cases progressed from younger age group (first 50 percentile cases) to old age group (last 50 percentile cases), the stone size progressively increased, while the fraction of preneoplastic and neoplastic cases markedly increased as well. So, it can be considered that with increasing age, gallstone in chronic cases of cholelithiasis may increase in size, causing continuous inflammation, which may lead to the metaplasia-dysplasia-carcinoma sequence in GB. Various studies have indicated that the progression from metaplasia to dysplasia takes three years, and from dysplasia to carcinoma, approximately ten years [
42]. The dysplasia-carcinoma sequence is an accepted carcinogenic pathway for GB cancer, a process that would require a period of approximately 10–15 years [
43]. In the current study, the average duration of cholelithiasis among carcinoma patients was approximately 15 months, with the maximum being 28 months in one case of carcinoma, whereas among all other cases of cholelithiasis, approximately 4 months was the average duration. The univariate analysis showed duration of symptom to be a significant risk factor for GB carcinoma. In current study, the duration of symptom was shorter among carcinoma patients, as compared to the 10–15 years involved in the dysplasia-carcinoma sequence. The Mishra et al. [
44] study of GB carcinoma risk factors in India concluded that low education level, the drinking of tap water, and multiparity to be associated with higher risk of GB carcinoma. Since the population in our study mostly belonged to the poor strata of society, they were very likely to be associated with all of the above-described risk factors. The simulatanous exposure to these risk factors may have accelerated the dysplasia carcinoma sequence, leading to neoplastic changes in a shorter duration of time. Moreover, due to the lower educational status and awareness, as well as poor access to health facilities due to meagre income level, they were very less likely to get any medical attention during initial episodes of cholecystitis, and ignore the initial period of disease, and hence received a delayed diagnoses for their cholelithiasis when preneoplastic changes may have already been progressing in GB mucosa. Serra et al. [
45] hypothesised that the logical consequence of a decrease in the cholecystectomy rate would result in an augmented number of gallstone carriers in the population, and hence older stones with an increased diameter, resulting in higher incidence and mortality rates from GB cancer.
The multivariate analysis of risk factors of GB carcinoma, which included patient age, GB wall thickness, duration of symptoms, number of stones, and size of stones, found that none of these factors had statistically significant association with GB carcinoma. However, the duration of symptom (
p = 0.056) and age (
p = 0.092) were found to have marginally insignificant
p-values, indicating they had a closer association with GB carcinoma, compared with the other risk factors. Moreover, conditional multivariate analysis for the duration of symptoms yielded significant
p-value (0.008). Scott et al. [
46] demonstrated age and symptomatic cholelithiasis to be associated with GB carcinoma in a multivariate analysis. Long-standing cholelithiasis tends to cause constant damage to the GB mucosa over time, therefore older patients presenting with long history of cholelithiasis have a higher risk of GB carcinoma. Although the sample size may be small, the incidence of 5% cases of GB carcinoma among cholecystectomy cases in the current study, along with the recent study by Sharma and Singh [
47] in Sri Guru Ramdass medical college, Amritsar, Punjab on GB cancer cases, highlights the magnitude of GB carcinoma disease affecting the residing population of the Punjab. Sharma and Singh [
47] restrospectively analysed 288 cases of GB carcinoma during the period 2010–2018. In this study, 66% cases had unresectable disease, and only 27 cases (9%) were able to survive to the last date of follow-up, hence portraying the high incidence and mortality caused by GB carcinoma. Just recently, there have been reports of a program of irreversible electroporation being initiated in PGI, Chandigarh for the late stage of GB carcinoma [
48].
Conclusion
While the current study initially showed age, gallstone number, gallstone size, and duration of symptom to be significant risk factors for GB carcinoma in univariate analysis, multivariate analysis was unable to display any of these risk factors as significant risk factors, probably due to the small sample size and uneven group distribution (GB carcinoma: 5 cases, other cases of cholelithiasis: 95) playing a part in the variance between the univariate and multivariate analysis results. However, the age and duration of symptoms had slightly marginally insignificant p-value, indicating their comparative closer association with GB carcinoma, while the conditional multivariate analysis for the duration of symptoms yielded significant p-value. This study sheds light on the rising incidence of cholelithiasis in the young population, and high rate of metaplasia, dysplasia, and carcinoma in the middle age and elderly population. Although individual analysis of gallstone size showed it to be a significant risk factor, multivariate analysis could not prove the same. Further research needs to be conducted to precisely define the sequence of events, as well as biochemical processes involved in mucosal damage leading to malignancy in gallstone disease, so that better judgement in the management of disease can be made. The limitations of this study were the smaller sample size and short follow-up period. Further, the patients mostly belonged to the economically poor strata of society, who, due to their meagre incomes and lower level of awareness, might be comparatively more exposed to adulterated consumables containing carcinogens, so the subject selection could have been more randomized to be more representative of the society. Still, further attention should be given to the rising cases of GB cancer cases in North India (particularly in the state of Punjab), to study its epidemiology and etiopathogenesis, so that corresponding proactive measures can be taken to control the disease.
Go to :






 PDF
PDF Citation
Citation Print
Print



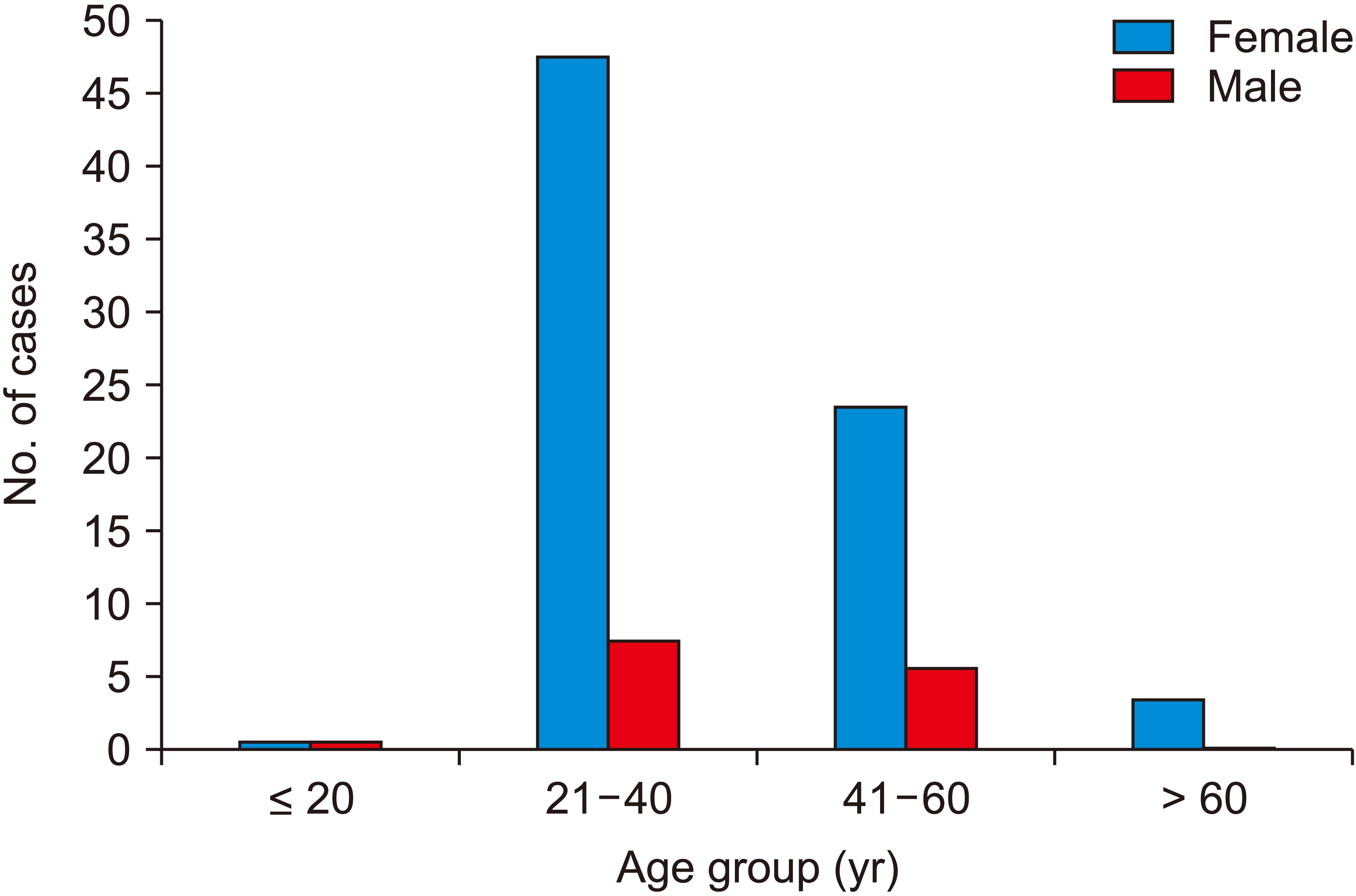



 XML Download
XML Download