Abstract
Backgrounds/Aims
Pancreaticoduodenectomy (PD) is commonly performed pancreatic procedure for tumors of periampullary region. Delayed gastric emptying (DGE) and pancreatic fistula are the most common specific complications following PD. DGE can lead to significant morbidity, resulting in prolonged hospital stay and increased cost. Various factors might influence the occurrence of DGE. We hypothesized that kinking of jejunal limb could be a cause of DGE post PD.
Methods
Antecolic (AC) and retrocolic (RC) side-to-side gastrojejunostomy (GJ) groups in classical PD were compared for the occurrence of DGE in a prospective study. All patients who underwent PD between April 2019 and September 2020 in a tertiary care center in south India were included in this study.
Go to : 
Pancreaticoduodenectomy (PD) for periampullary tumors is a complicated procedure that carries a high morbidity. Delayed gastric emptying (DGE) is the most common specific as well as avoidable cause of morbidity [1]. Several articles, randomized controlled trials (RCTs), and meta-analyses have been published on the cause of this avoidable morbidity [2-10]. Unfortunately, none of the aforementioned studies conclusively found an avoidable cause.
Definition of DGE provided by the International Study Group on Pancreatic Surgery (ISGPS) was used for this study [11]. DGE causes including loss of gastric antral pump due to antral vagotomy, relative ischemia of gastric antrum, and postoperative complications (such as postoperative pancreatitis or pancreatic or biliary or enteric leaks) have been suggested. Several authors have published their efforts to mitigate DGE by modifying intraoperative procedures such as classic versus pylorus-preserving [12], antecolic (AC) versus retrocolic (RC), pancreaticogastrostomy versus pancreaticojejunostomy [13], duodenal preservation [14], right gastric artery preservation [14], and pancreaticojejunostomy stent placement [2]. However, after all these efforts, the most effective technique to mitigate DGE after PD is still debatable. The route of reconstruction with respect to the transverse colon, AC vs. RC route, was focused in this study.
We theorized that, in side-to-side gastrojejunostomy (GJ) of classic PD, RC anastomosis would have less angulation and hence more gravity-dependent flow of stomach contents compared to AC anastomosis, which might help decrease DGE.
Go to : 
A prospective comparative study was conducted in the Department of Surgical Gastroenterology at a tertiary care hospital between April 2019 and September 2021, including all patients planned for PD for any indication. Patients who had previously undergone any gastric or small bowel surgeries and those who were on prolonged postoperative mechanical ventilator were excluded from this study. A prospective study was conducted after obtaining approval from institutional research and ethics committee vide institutional ethical committee number 946. Informed written consent was taken from each patient before participating in this study.
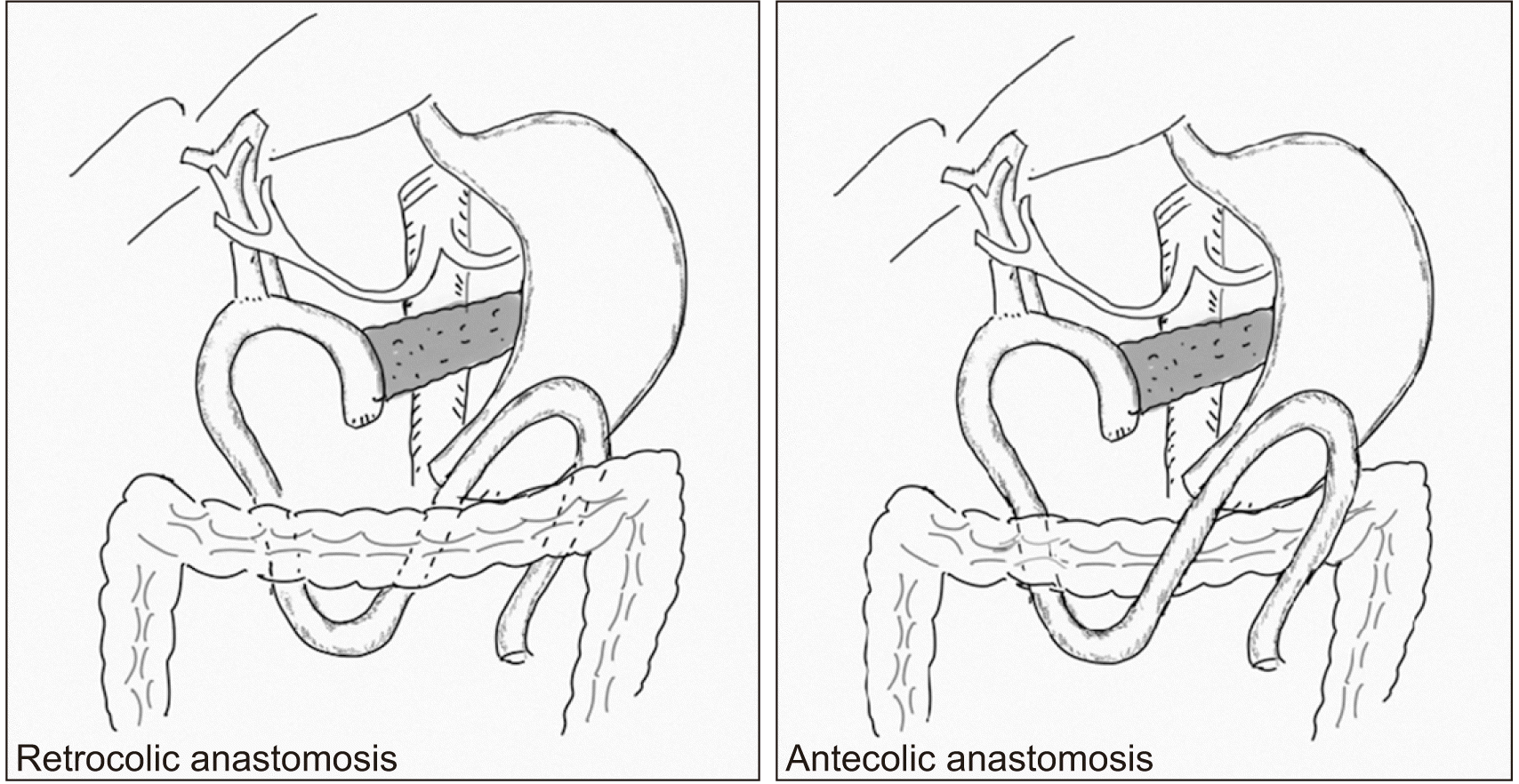
Surgical Technique and Data Collection: After resection of the specimen, pancreaticojejunostomy (duct-to-mucosa technique) and hepaticojejunostomy were completed. A RC limb of the jejunum (through mesocolon to the right of the middle colic artery) was utilized for pancreatic and biliary anastomosis. Patients were randomized after completion of resection and after confirming the feasibility of both techniques in each patient to either AC or RC gastrojejunal anastomosis group based on ‘blocks of four’ method with an allocation ratio of 1:1. AC or RC GJ was done approximately 45 cm from the hepaticojejunostomy in two layers spanning 5 to 6 cm using 2/0 polyglactin 910 and 2/0 polypropylene. In the RC group, the same loop of the jejunum was brought up to the stomach through the transverse mesocolon through a separate rent in the mesocolon to the left of the middle colic artery (Fig. 1). Two abdominal drains were placed, one near the pancreaticojejunostomy and other near the hepaticojejunostomy. Feeding jejunostomy was done in all cases.
Proton pump inhibitors were administered to all patients postoperatively. Nasogastric tube (NGT) was removed preferably on day 1 or 2 once the NGT output was less than 500 mL. NG was reinserted if patient had vomiting. Feeds through feeding jejunostomy tube were started on day 2. Oral liquids were started after NGT removal and solid food was started if patient could tolerate liquids. Drain fluid amylase concentration analysis was done on day 3. Drains were removed after postoperative day (POD) 3 if drain fluid amylase was normal and no biliary fistula was evident.
The day of removal of NGT and the day of resumption of liquid and solid diet were noted. Both drains’ fluid amylase levels on POD 3 were measured. Volumes of both drains’ effluents were also recorded postoperatively.
A prospective comparative study was conducted. Normally distributed continuous variables were compared using the unpaired t-test, whereas the Mann–Whitney U test was used for those variables that were not normally distributed. Categorical variables were analyzed using either the chi-square test or Fisher’s exact test. For all statistical tests, a p-value of less than 0.05 was taken to indicate a significant difference. All calculations were performed using SPSS ver. 20.0 (IBM Corp.).
Go to : 
A total of 73 patients were included in the study. All patients underwent an explorative laparotomy. Of these, 11 patients were excluded before randomization because these patients had an unresectable disease. Sixty-two patients were randomized into two groups: AC (32 patients, 51.6%) and RC (30 patients, 48.4%) anastomosis. Males accounted for 71.9% (23/32) in the AC group and 70.0% (21/30) in the RC group (p = 0.157). Patient demographics, baseline investigations, ASA grade, intraoperative characteristics, and postoperative complications were comparable in both groups (Table 1).
Clinicodemographic and perioperative details
Thirty-one (50.00%) of 62 patients had NGT removed before or on day 3. The median day of NGT removal was the sixth POD in AC and the third POD in RC, showing a significant difference between the two groups (p = 0.006). The percentage of patients who needed reinsertion of the NGT was not significantly different between the two groups (4 patients in AC and 2 patients in RC group; p = 0.672). Postoperative endoscopy or contrast study was done in 6 patients in AC and 5 patients in RC group with a p-value of > 0.999. Re-intervention was required in 3 patients (1 in the AC group and 2 in the RC group, p = 0.607). The median day of solid food intake was the ninth POD in the AC group and seventh POD in the RC group, showing a significant difference between the two groups (p < 0.001). Cumulative incidence of DGE in the entire study population was 50%. DGE was present in 23 (71.9%) patients in the AC group and 8 (26.7%) patients in the RC group, showing a significant difference between the two (p = 0.001) (Table 2).
Delayed gastric emptying
Distribution among grades of DGE was also significant. Grade A DGE and Grade B DGE were more in the AC group. Grade C DGE was more in the RC group, although the difference between the two was not statistically significant (p = 0.189). Median postoperative hospital stay was 11 days in the AC group and 9 days in the RC group, showing a significant difference (p = 0.004). Postoperative complications such as postoperative pancreatic fistula (POPF), post pancreatectomy hemorrhage, surgical site infection, and postoperative mortality were comparable between the two groups (Table 3).
Go to : 
In the present study, 62 patients were randomized into AC (n = 32) and RC (n = 30) groups. Overall incidence of DGE in this study was 50%, with clinically significant DGE (ISGPS class B and C) accounting for 20.96%. The incidence of DGE was significantly higher in the AC group (71.9% vs. 26.7%; p = 0.001).However, POPF, PPH, SSI, and mortality of the two groups were similar to each other. Median hospital stay was lower in the RC group (9 days vs. 11 days; p = 0.004).
Technical causes of DGE have been studied thoroughly in the literature. Significant edema or kinking at GJ at either the afferent or efferent limb might be a factor in the development of DGE. Several anastomosis methods such as AC/RC end to side or side to side in pylorus-preserving/classical PD have been tried. Most of those studies aimed to find a cause of DGE. However, none of the preventable causes described in the literature has been proven to be able to prevent DGE except a straighter and gravity dependant anastomosis [4,15,16]. The present study tried to find a suitable anastomosis in classical PD for side-to-side GJ.
Although several studies have published conflicting results for gastroenteric anastomosis, few of them support AC anastomosis in end to side GJ in pylorus-preserving PD. It has been proposed that vertical anastomosis with less angulation can cause less DGE. Kurahara et al. (2011) [9] and Sahora et al. (2015) [16] in their respective studies have proven the benefit of AC end to side anastomosis over RC in pylorus-preserving PD. However, Oida et al. (2012) [10] have retrospectively analyzed 42 patients of modified subtotal stomach-preserving PD and found that RC anastomosis is better. Hu et al. [17] in 2014, Bell et al. [18] in 2015, Imamura et al. [19] in 2014, Zhou et al. [20] in 2015, Hanna et al. [21] in 2016, and Joliat et al. [22] in 2016 published meta-analysis and found conflicting results.
As all different studies have performed different types of anastomosis in their studies, these studies were not comparable. Changing the anatomy of the anastomosis will result in difference in gastric emptying. In side-to-side GJ, gastric emptying is gravity dependent, so a straighter and dependent anastomosis is preferred.
As this study involved only side-to-side anastomosis, a broader and multicenter study with different types of anastomosis is required to test this hypothesis. However, this study can provide a roadmap to find the cause of DGE in PD.
One major limitation of this study was that ERAS protocol was not adapted for the removal of NGT before the end of anesthesia. According to recent ERAS guidelines by Melloul et al. [23] in 2020, NGT placed during surgery should be removed before the end of the anesthesia. Cao et al. [24] in 2019 stated in a meta-analysis of 3,387 patients that pre-emptive use of NGT postoperatively did not improve outcomes. In addition, differences in rates of DGE were not reduced in the ERAS group. There were no significant differences in different grades of DGE between the two groups.
To conclude, delayed gastric emptying can cause significant morbidity following PD. Route of reconstruction of GJ can influence the occurrence of DGE. RC reconstruction of side-to-side GJ has less DGE than AC reconstruction. It is also associated with a shorter hospital stay in classic PD.
Go to : 
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: All authors. Data curation: All authors. Methodology: All authors. Visualization: All authors. Writing - original draft: LA. Writing - review & editing: All authors.
Go to : 
REFERENCES
1. Akizuki E, Kimura Y, Nobuoka T, Imamura M, Nagayama M, Sonoda T, et al. 2009; Reconsideration of postoperative oral intake tolerance after pancreaticoduodenectomy: prospective consecutive analysis of delayed gastric emptying according to the ISGPS definition and the amount of dietary intake. Ann Surg. 249:986–994. DOI: 10.1097/SLA.0b013e3181a63c4c. PMID: 19474680.
2. Parmar AD, Sheffield KM, Vargas GM, Pitt HA, Kilbane EM, Hall BL, et al. 2013; Factors associated with delayed gastric emptying after pancreaticoduodenectomy. HPB (Oxford). 15:763–772. DOI: 10.1111/hpb.12129. PMID: 23869542. PMCID: PMC3791115.
3. Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakagawa N, et al. 2008; An antecolic Roux-en Y type reconstruction decreased delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. J Gastrointest Surg. 12:1081–1086. DOI: 10.1007/s11605-008-0483-1. PMID: 18256885.
4. Hartel M, Wente MN, Hinz U, Kleeff J, Wagner M, Müller MW, et al. 2005; Effect of antecolic reconstruction on delayed gastric emptying after the pylorus-preserving Whipple procedure. Arch Surg. 140:1094–1099. DOI: 10.1001/archsurg.140.11.1094. PMID: 16301447.
5. Tani M, Terasawa H, Kawai M, Ina S, Hirono S, Uchiyama K, et al. 2006; Improvement of delayed gastric emptying in pylorus-preserving pancreaticoduodenectomy: results of a prospective, randomized, controlled trial. Ann Surg. 243:316–320. DOI: 10.1097/01.sla.0000201479.84934.ca. PMID: 16495694. PMCID: PMC1448934.
6. Chijiiwa K, Imamura N, Ohuchida J, Hiyoshi M, Nagano M, Otani K, et al. 2009; Prospective randomized controlled study of gastric emptying assessed by (13)C-acetate breath test after pylorus-preserving pancreaticoduodenectomy: comparison between antecolic and vertical retrocolic duodenojejunostomy. J Hepatobiliary Pancreat Surg. 16:49–55. DOI: 10.1007/s00534-008-0004-3. PMID: 19083149.
7. Gangavatiker R, Pal S, Javed A, Dash NR, Sahni P, Chattopadhyay TK. 2011; Effect of antecolic or retrocolic reconstruction of the gastro/duodenojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a randomized controlled trial. J Gastrointest Surg. 15:843–852. DOI: 10.1007/s11605-011-1480-3. PMID: 21409601.
8. Tamandl D, Sahora K, Prucker J, Schmid R, Holst JJ, Miholic J, et al. 2014; Impact of the reconstruction method on delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: a prospective randomized study. World J Surg. 38:465–475. DOI: 10.1007/s00268-013-2274-4. PMID: 24121364.
9. Kurahara H, Shinchi H, Maemura K, Mataki Y, Iino S, Sakoda M, et al. 2011; Delayed gastric emptying after pancreatoduodenectomy. J Surg Res. 171:e187–e192. DOI: 10.1016/j.jss.2011.08.002. PMID: 22001182.
10. Oida T, Mimatsu K, Kano H, Kawasaki A, Fukino N, Kida K, et al. 2012; Antecolic and retrocolic route on delayed gastric emptying after MSSPPD. Hepatogastroenterology. 59:1274–1276. DOI: 10.5754/hge10113. PMID: 22580680.
11. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. 2007; Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 142:761–768. DOI: 10.1016/j.surg.2007.05.005. PMID: 17981197.
12. van Berge Henegouwen MI, van Gulik TM, DeWit LT, Allema JH, Rauws EA, Obertop H, et al. 1997; Delayed gastric emptying after standard pancreaticoduodenectomy versus pylorus-preserving pancreaticoduodenectomy: an analysis of 200 consecutive patients. J Am Coll Surg. 185:373–379. DOI: 10.1016/S1072-7515(01)00944-9. PMID: 9328386.
13. Wellner UF, Sick O, Olschewski M, Adam U, Hopt UT, Keck T. 2012; Randomized controlled single-center trial comparing pancreatogastrostomy versus pancreaticojejunostomy after partial pancreatoduodenectomy. J Gastrointest Surg. 16:1686–1695. DOI: 10.1007/s11605-012-1940-4. PMID: 22744638.
14. Müller MW, Friess H, Beger HG, Kleeff J, Lauterburg B, Glasbrenner B, et al. 1997; Gastric emptying following pylorus-preserving Whipple and duodenum-preserving pancreatic head resection in patients with chronic pancreatitis. Am J Surg. 173:257–263. DOI: 10.1016/S0002-9610(96)00402-3. PMID: 9136776.
15. Masui T, Kubora T, Nakanishi Y, Aoki K, Sugimoto S, Takamura M, et al. 2012; The flow angle beneath the gastrojejunostomy predicts delayed gastric emptying in Roux-en-Y reconstruction after distal gastrectomy. Gastric Cancer. 15:281–286. DOI: 10.1007/s10120-011-0107-4. PMID: 22041869.
16. Sahora K, Morales-Oyarvide V, Thayer SP, Ferrone CR, Warshaw AL, Lillemoe KD, et al. 2015; The effect of antecolic versus retrocolic reconstruction on delayed gastric emptying after classic non-pylorus-preserving pancreaticoduodenectomy. Am J Surg. 209:1028–1035. DOI: 10.1016/j.amjsurg.2014.04.015. PMID: 25124295.
17. Hu HL, Zhou XD, Zhang Q, Shi X. 2014; Factors influencing delayed gastric emptying after pancreaticoduodenectomy - a meta-analysis. Hepatogastroenterology. 61:1539–1545. PMID: 25436339.
18. Bell R, Pandanaboyana S, Shah N, Bartlett A, Windsor JA, Smith AM. 2015; Meta-analysis of antecolic versus retrocolic gastric reconstruction after a pylorus-preserving pancreatoduodenectomy. HPB (Oxford). 17:202–208. DOI: 10.1111/hpb.12344. PMID: 25267428. PMCID: PMC4333780.
19. Imamura N, Chijiiwa K, Ohuchida J, Hiyoshi M, Nagano M, Otani K, et al. 2014; Prospective randomized clinical trial of a change in gastric emptying and nutritional status after a pylorus-preserving pancreaticoduodenectomy: comparison between an antecolic and a vertical retrocolic duodenojejunostomy. HPB (Oxford). 16:384–394. DOI: 10.1111/hpb.12153. PMID: 23991719. PMCID: PMC3967891.
20. Zhou Y, Lin J, Wu L, Li B, Li H. 2015; Effect of antecolic or retrocolic reconstruction of the gastro/duodenojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a meta-analysis. BMC Gastroenterol. 15:68. DOI: 10.1186/s12876-015-0300-8. PMID: 26076690. PMCID: PMC4467059.
21. Hanna MM, Tamariz L, Gadde R, Allen C, Sleeman D, Livingstone A, et al. 2016; Delayed gastric emptying after pylorus preserving pancreaticoduodenectomy-does gastrointestinal reconstruction technique matter? Am J Surg. 211:810–819. DOI: 10.1016/j.amjsurg.2015.10.015. PMID: 26792273.
22. Joliat GR, Labgaa I, Demartines N, Schäfer M, Allemann P. 2016; Effect of antecolic versus retrocolic gastroenteric reconstruction after pancreaticoduodenectomy on delayed gastric emptying: a meta-analysis of six randomized controlled trials. Dig Surg. 33:15–25. DOI: 10.1159/000441480. PMID: 26566023.
23. Melloul E, Lassen K, Roulin D, Grass F, Perinel J, Adham M, et al. 2020; Guidelines for perioperative care for pancreatoduodenectomy: enhanced recovery after surgery (ERAS) recommendations 2019. World J Surg. 44:2056–2084. DOI: 10.1007/s00268-020-05462-w. PMID: 32161987.
24. Cao Y, Gu HY, Huang ZD, Wu YP, Zhang Q, Luo J, et al. 2019; Impact of enhanced recovery after surgery on postoperative recovery for pancreaticoduodenectomy: pooled analysis of observational study. Front Oncol. 9:687. DOI: 10.3389/fonc.2019.00687. PMID: 31417868. PMCID: PMC6683725.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download