Abstract
Backgrounds/Aims
Proximal splenorenal shunt (PSRS) is a commonly performed procedure to decompress portal hypertension, in patients with refractory variceal bleed, especially in non-cirrhotic portal hypertension (NCPH). If conventional methods are hindered by any technical or pathological factors, alternative surgical techniques may be required. This study analyzes the effectiveness of various unconventional shunt surgeries performed for NCPH.
Methods
A retrospective analysis of NCPH patients who underwent unconventional shunt surgeries during the period July 2011 to June 2022 was conducted. All patients were followed up for a minimum of 12 months with doppler study of the shunt to assess shunt patency, and upper gastrointestinal endoscopy to evaluate the regression of varices.
Results
During the study period, 130 patients underwent shunt surgery; among these, 31 underwent unconventional shunts (splenoadrenal shunt [SAS], 12; interposition mesocaval shunt [iMCS], 8; interposition PSRS [iPSRS], 6; jejunal vein-cava shunt [JCS], 3; left gastroepiploic–renal shunt [LGERS], 2). The main indications for unconventional shunts were left renal vein aberration (SAS, 8/12), splenic vein narrowing (iMCS, 5/8), portalhypertensive vascular changes (iPSRS, 6/6), and portomesenteric thrombosis (JCS, 3/3). The median fall in portal pressure was more in SAS (12.1 mm Hg), and operative time more in JCS, 8.4 hours (range, 5–9 hours). During a median follow-up of 36 months (6–54 months), shunt thrombosis had been reported in all cases of LGERS, and less in SAS (3/12). Variceal regression rate was high in SAS, and least in LGERS. Hypersplenism had reversed in all patients, and 6/31 patients had a recurrent bleed.
Go to : 
In developing countries, 20%–30% of cases of portal hypertension were constituted by non-cirrhotic portal hypertension (NCPH) [1], which commonly causes variceal bleeding. Long-term portal hypertension poses significant morbidities, such as hypersplenism, portal biliopathy, growth failure, and ectopic varices [2]. Portosystemic shunt surgery is frequently performed to decompress the portal system in patients with variceal bleed, refractory to medical and endoscopic management, especially in NCPH. Shunt surgery remains a one-time treatment procedure with durable, long-term efficacy in preventing variceal rebleeding, and preventing morbidities associated with NCPH [3]. Proximal splenorenal shunt (PSRS) is the commonly used procedure in NCPH, especially in patients with a large spleen. However, in some patients, etiological, pathological and technical factors increase the difficulty of performing conventional PSRS, and hence different types of unconventional shunt surgeries may be required. Possible alternatives for conventional shunt surgery were the use of PTFE or Dacron graft in interposition between conduits (interposition PSRS [iPSRS] and interposition mesocaval shunt [iMCS]), and the use of other conduits, like adrenal vein, first jejunal, and left gastroepiploic vein. This study analyzes the experience of various unconventional shunt surgeries for the management of NCPH.
Go to : 
A retrospective analysis was performed of NCPH patients who underwent unconventional shunt surgeries between July 2011 and June 2022. Preoperatively, patients were assessed with Doppler study of the portospleno mesenteric system and upper GI-scopy to evaluate varices, in addition to haematological investigation and liver function test. All patients received a triple vaccine prior to operation (pneumococcal, meningococcal, and Haemophilus influenzae). Pre- and post-shunt portal pressure was measured in omental vein, intraoperatively. Splenectomy was performed either by the early arterial ligation–late mobilization, or early mobilisation–late arterial ligation methods. All the patients were started on heparin intraoperatively after the shunt was performed. This was continued in the postoperative period, and bridged with an oral anticoagulant on postoperative day (POD) 3. Postoperative morbidities, classified according to the Clavien–Dindo classification, were analyzed among each surgical group. A routine follow-up of a Doppler study and upper gastrointestinal endoscopy were performed at 3, 6, and 12 months to assess shunt patency and regression of varices, respectively. Oesophageal varices were graded in the range (1 to 4) using Conn’s criteria.
Age, sex, cause of portal hypertension, clinical presentation, hematological parameters, symptomatic hypersplenism, intraoperative findings, and postoperative complications were tabulated and analyzed. Hypersplenism was considered when splenomegaly was associated with the reduction of one or more cell lineage in blood, like anemia with hemoglobin < 8 g/dL, total leucocytes < 3,500/mm3, or low platelet count of < 1.5 lac/mm3. Symptomatic hypersplenism was weighed with symptoms due to anemia, recurrent infection, and bleeding episodes, along with the above criteria [4].
The study was approved by the ethical committee of the JIPMER (JIP/IEC/2014/10/478). The informed consent was waived.
Splenoadrenal shunt
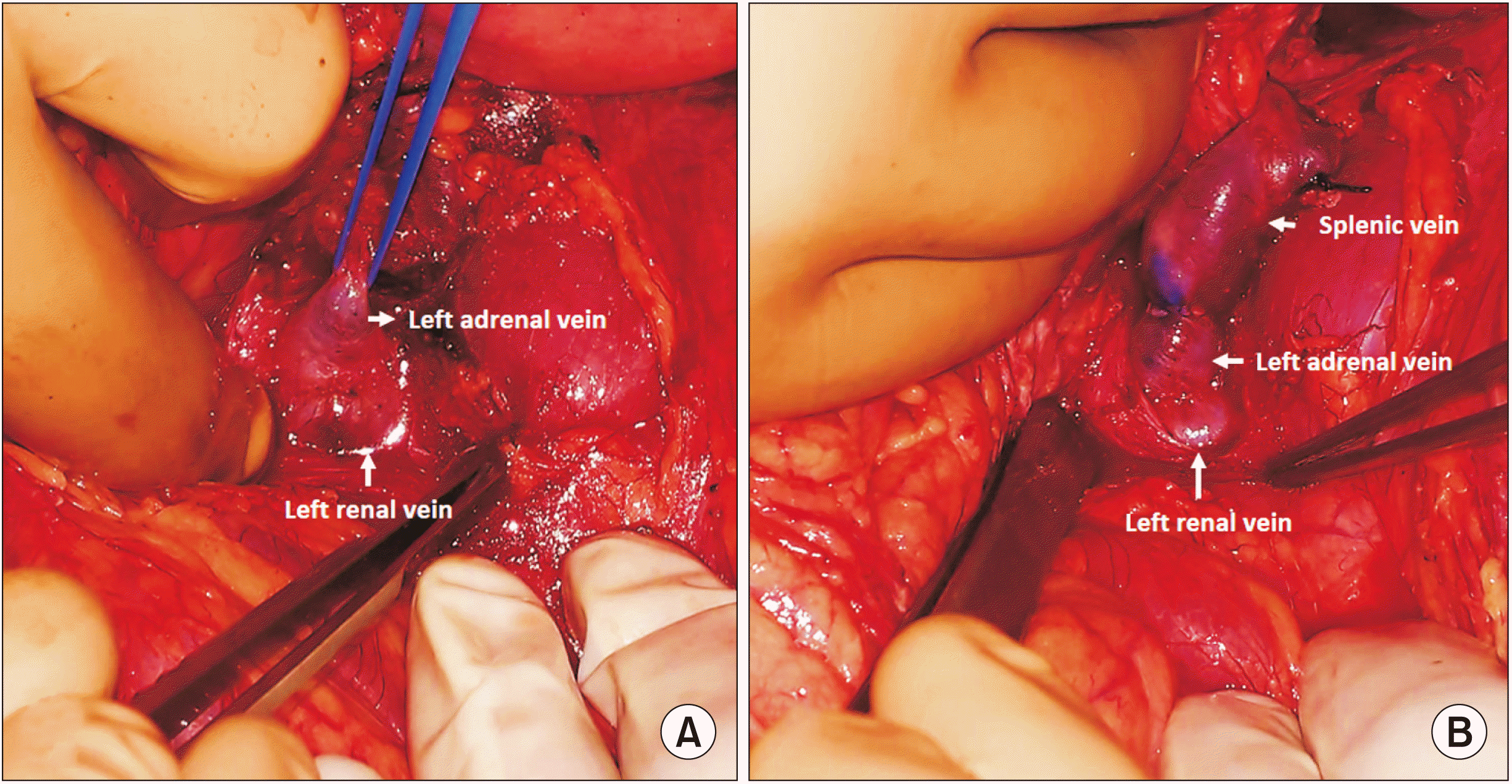
Splenoadrenal shunt (SAS) was performed in a supine position with a trap door incision. After splenectomy was performed, Gerota’s fascia was dissected, and the left kidney was exposed at the medial aspect. The left renal vein was identified and dissected. The left adrenal vein was isolated, and an end-to-end shunt between the splenic and renal end of the adrenal vein was performed with 6-0 prolene continuously (Fig. 1). The end-to-side anastomosis was performed in cases with a disparity in shunt vessel diameter.
Interposition mesocaval shunt
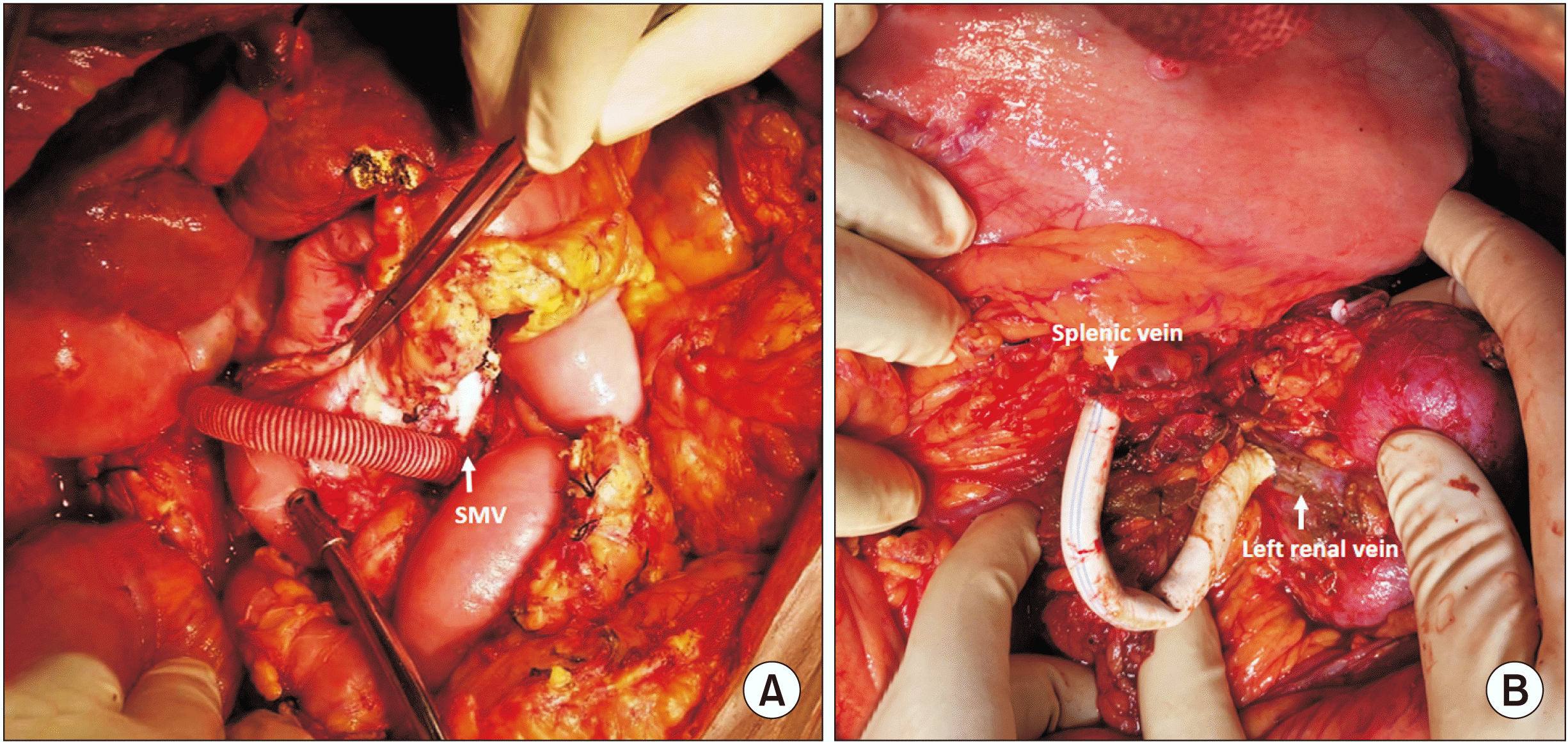
This shunt surgery was performed in a supine position and midline incision. Kocherisation was done to expose the superior mesenteric vein (SMV). This was facilitated by partial mobilization of the hepatic flexure. The middle colic vein was identified and ligated to obtain sufficient length for a shunt. SMV was identified and looped. Similarly, inferior vena cava (IVC) was freed of adhesions, and was looped. Dacron ringed/PTFE 8-mm graft was primed with heparinized saline and the patient’s blood. First, the graft was anastomosed to IVC using the Satinsky side biting clamp by prolene 6-0. Subsequently, the graft was anastomosed to SMV in a similar manner. SMV anastomosis was completed after releasing air and a small amount of blood. Trial declamping was done, and good flow was noted. IVC clamp was removed first, followed by the SMV clamp (Fig. 2A).
Interposition proximal splenorenal shunt
Position, incision, and initial steps were similar to the spleno adrenal shunt. Retroperitoneum was dissected at the level of the left renal vein, and Gerota’s fascia was opened to trace the left renal vein. The left renal vein was identified and dissected to facilitate the anastomosis. The left gonadal vein was identified and ligated. A 10 cm × 8 mm PTFE graft/Dacron was used for the shunt anastomosis. The PTFE graft was initially anastomosed to the left renal vein in an end-to-side fashion using 6-0 prolene, aligning the blue markings to the left edge of the venotomy. The clamps were released, and hemostasis was ensured. The splenic vein was cut sharp at the level of planned anastomoses, after clamping it proximally. Trial declamping was done, and good flow was confirmed from the splenic vein. The splenic vein was anastomosed to the PTFE graft in an end-to-end fashion using 6-0 prolene in a continuous manner (Fig. 2B).
Jejunocaval shunt
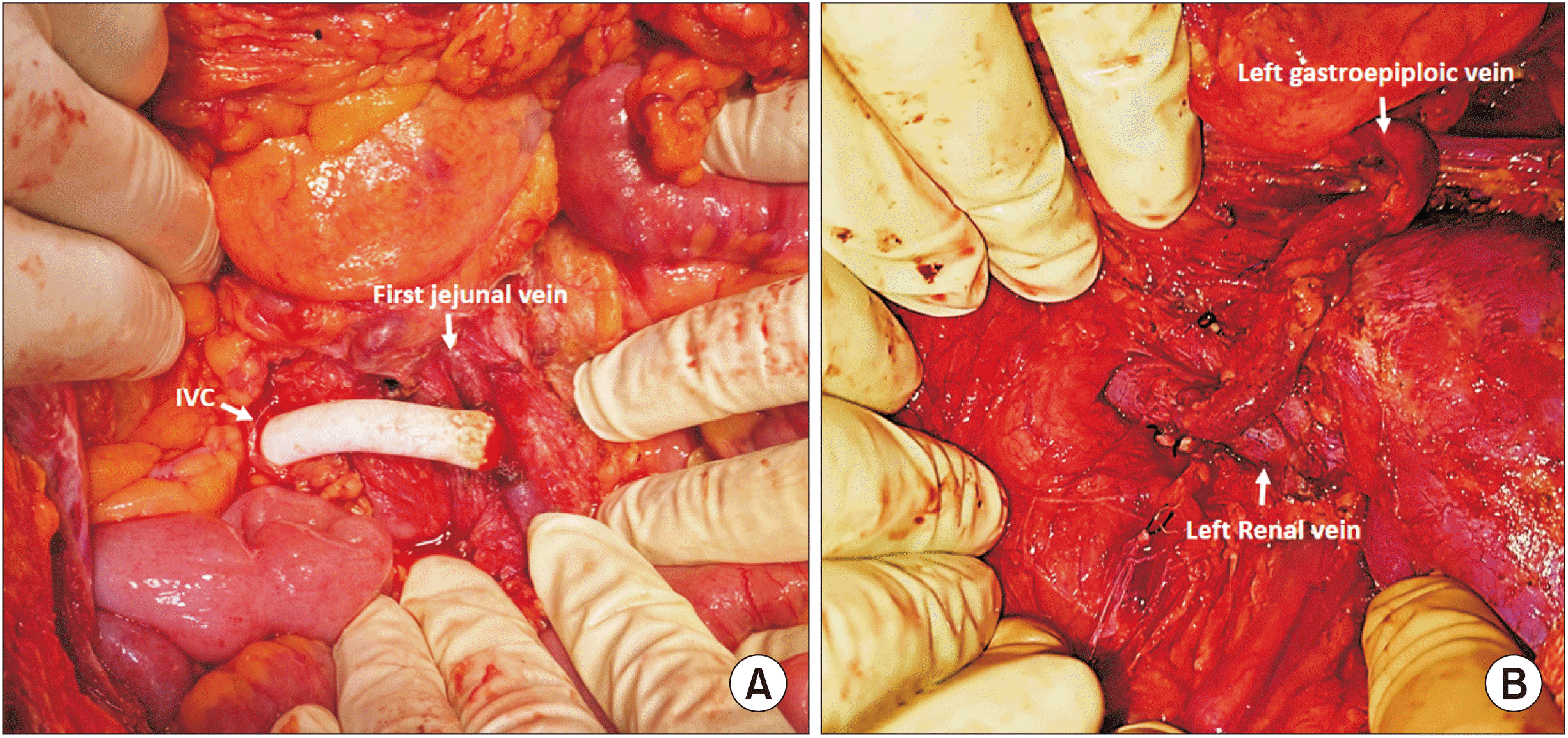
The initial steps were similar to the iMCS surgery. The jejunal vein was skeletonized, and looped with a vessel loop. The caecum and ascending colon were mobilized from the right lateral abdominal wall (Cattell Braasch), and infrarenal IVC was exposed. IVC was looped, and a side-biting (Satinsky) vascular clamp was applied. PTFE graft of size 8 mm × 10 cm length was flushed with heparinized blood. Stay suture was taken over IVC using prolene 5-0. Venotomy was performed using Potts scissors. PTFE graft was sutured to the IVC using 6-0 prolene with a growth factor. A spoon clamp was applied over the graft, and the Satinsky clamp over IVC was released. The graft was brought into the infracolic compartment through a mesocolic window created in the ascending colon. A side-biting clamp was applied over the jejunal vein, and venotomy was done. PTFE graft was sutured to the jejunal vein using 6-0 prolene. Spoon clamp was released (Fig. 3A).
Left gastroepiploic–renal vein shunt
Surgery was performed in a supine and left trap door incision. The splenic artery was identified and ligated. Splenectomy was performed. The left gastroepiploic vein was skeletonized, and adequate length dissected out. Retroperitoneum was dissected at the level of the left renal vein, and Gerota’s fascia was opened to trace the left renal vein. The anterior surface of the left kidney and its hilum were separated from the surrounding fat. The left renal vein was identified and dissected. The anterior surface of the splenic vein was cleared for anastomosis. The left gastroepiploic vein was cut sharp at the level of planned anastomosis, after clamping it proximally. Trial declamping was done. The left gastroepiploic vein was anastomosed to the left renal vein in an end-to-side fashion using a 6-0 prolene double-armed suture in a continuous manner (Fig. 3B).
Statistical analysis was performed using SPSS version 21.0 (IBM Corp.). Medians, ranges, and frequencies were used to describe the data.
Go to : 
During the study period, 130 patients with NCPH underwent shunt surgeries; 31 underwent unconventional shunts (16 extrahepatic portal vein obstruction [EHPVO], and 15 non-cirrhotic portal vein fibrosis [NCPF]). There were 21 females and 18 patients who presented with upper gastrointestinal bleed, while 3 came with abdomen fullness and pain; other presentations were menorrhagia, epistaxis, recurrent infection. Preoperative evaluation revealed that all patients had features of hypersplenism; among them, 20 had symptomatic hypersplenism. The types and frequency of unconventional shunt procedure were as follow: 1) SAS: 12; 2) iMCS: 8; 3) iPSRS: 6; 4) jejunal vein-caval shunt (JCS): 3; 5) left gastroepiploic–renal shunt (LGERS): 2. Table 1 illustrates the demographics of each procedure.
Unconventional shunts-demographics, intraoperative and postoperative findings
Among 31 unconventional shunt surgeries, 12 patients underwent splenoadreanal shunt (6 NCPF and 6 EHPVO). The main indication for splenoadreanal shunt was left renal vein aberration; 8 patients had an anomalous union of the superior and inferior polar branches. Splenoadreanal anastomosis was performed in an end-to-end manner in 10 patients, and side-to-side anastomosis in 2 patients. The median shunt diameter was 8 mm with a range of 5–15 mm, and the median fall in portal hypertension was 12.1 mm Hg. The median operative time was 5 hours (3–6.5 hours), and median blood loss was 210 mL (100–330 mL). During a median follow-up of 30 months (9–46 months), hypersplenism was reversed in all patients, and variceal regression was noted in 10 patients; 3 patients had shunt thrombosis, and 2 patients had a recurrent bleed.
Among 8 patients, 5 were EHPVO, and the remaining were NCPF. Splenic vein narrowing (5 patients) was the common indication for mesocaval shunt, 2 others had dense adhesion of the hilum, and 1 had splenic vein thrombosis. Interposition was accomplished using PTFE and Dacron grafts. Eight patients received an 8-mm PTFE graft, 2 received an 8-mm Dacron graft, and 1 received a 10-mm Dacron graft. The median fall in portal hypertension after shunt surgery was 10.16 mm Hg, and the median operative time was 6.5 hours (5–7 hours). Median blood loss was 350 mL (260–750 mL), and 3 patients developed ascites in immediate postoperative periods, which were managed conservatively with diuretics. During a median follow-up of 32 months (24–45 months), variceal regression occurred in all patients; 4 had shunt thrombosis, of these, 2 patients had recurrent bleed.
Here, 5 cases were NCPF, and 1 was EHPVO. Portal hypertensive splenic vein changes were a common indication for iPSRS, and were present in each case. Excision of focal vascular changes led to inadequate length of the splenic vein, and hence interposition graft was performed. Here, PTFE 8-mm graft was used in 3 cases, and 7-mm in 2 cases, while Dacron 10-mm graft was used in 1 case. The median fall in portal hypertension was 10.12 mm Hg, with a median operative time of 6 hours (4–7.5 hours), and median blood loss of 280 mL (100–1,200 mL). One patient developed grade I hepatic encephalopathy, which was managed with antiencepholpathic measures. During a median follow-up of 38 months (3–52 months), variceal regression occurred in 5 patients, shunt thrombosis in 2 patients, and among them, 1 had recurrent bleeding. Hypersplenism was reversed in all patients.
Among 3 patients who underwent interposition first JCS, 2 were EHPVO, and 1 had NCPF. All patients had portal mesenteric thrombosis, which was the main indication for using the jejunal vein as a conduit for the shunt. PTFE 8-mm graft was used in all cases, and the median fall in portal hypertension was 8.45 mm Hg. The median operative time was 8.4 hours (5–9 hours), and blood loss was 320 mL. One had developed ascites, which was managed medically. On follow-up with a median duration of 33 months (2–40 months), hypersplenism was reversed, and varices were regressed in all patients. One developed shunt thrombosis, and none had a recurrent bleed.
Two patients who had EHPVO, with portomesentric thrombosis with no shuntable jejunal vein, underwent a LGERS. The shunt diameter was 4 mm in both cases, and the mean fall in portal hypertension was 7.5 mm Hg. The mean operative time was 7.5 hours, and blood loss was 340 mL. During a follow-up of 24 months, hypersplenism and varices resolved in 1 patient, shunt thrombosis occurred in both patients, and among them, 1 had a recurrent bleed.
Table 2 demonstrates operative details.
Unconventional shunt: long-term follow-up outcomes
Go to : 
NCPH, a common problem in developing countries, does not have a well-demarked severity classification like cirrhosis. Patients with recurrent variceal bleed, symptomatic hypersplenism, and other long-term complications are considered high-risk groups. Medical and endoscopic primary prophylaxis and management are well-defined in the literature to reduce portal hypertension. Shunt surgeries in NCPH are indicated when medical management has failed, and they are a durable and effective method.
Blakemore and Lord performed splenorenal shunt surgery with an end-to-end anastomosis after nephrectomy [5]. In 1947, Linton et al. [5] published a paper on five patients who underwent PSRS without nephrectomy (end-to-side anastomosis). In 1989, Mazariegos and Reyes [6] in Pittsburgh described a modification of the splenorenal shunt by using the adrenal vein as conduits, and reported a case series of 12 patients in 1998 with long-term shunt patency and minimal morbidity.
SASs were used as selective distal splenorenal shunt (DSRS) or nonselective shunt (PSRS) modifications. Pittsburg study was a modification of DSRS, and our series were modifications of PSRS to also address hypersplenism. Gu et al. [7] concluded that splenoadreanal shunt was non-inferior to PSRS in terms of reduction in post-shunt portal hypertension, long-term patency, variceal regression, and reversal of hypersplenism. Anatomical and pathological factors in the splenic vein, such as inadequate diameter, length, unfavourable angulation, and increased fibrosis around the splenic vein in the pancreatic bed, led to unsatisfactory shunt, which increases the risk of shunt thrombosis [8]. Left renal vein aberrations (anomalous union of superior and left inferior polar branches of the renal vein) were the most common indication of SAS in our study. Gupta et al. [8] described the benefits of using adrenal veins as conduits as i) natural conduits were superior to prosthetic graft, with reduced risk of infection and thrombosis, ii) the left adrenal vein will be exposed during dissection and mobilization of the left renal vein, iii) the drainage of the adrenal vein into the left renal vein with an optimal anatomic angle, which favoured a tension-free anastomosis, and iv) it deflected the need for a vascular anastomosis directly into the renal vein by avoiding renal vein clamping, which has the potential to cause thrombosis postoperatively. Postoperative ascites were reported in 2 patients, managed with diuretics, and comparable with other case series.
Reynolds and Southwick performed the first interposition shunt between the portal vein and IVC using an azygous vein in 1953, and the first prosthetic interposition portocaval shunt was conducted in 1962 [9]. In the 1970s, Drapanas [9] popularised the iMCS and its hemodynamics. Mesocaval shunt with side-to-side interposition graft size > 12 mm diameter diverts the whole portal flow, while 8–10 mm diameter maintains some prograde flow [10]. Drapanas et al. [10] performed mesocaval shunt surgery on 25 patients, and the most troublesome challenge faced during surgery was the early bifurcation of the SMV. Post-shunt portal pressure decreased by more than 50% in all patients. On a median follow up of 42 months, there was no evidence of rebleed, hepatic encephalopathy, or any hepatic impairment [9]. In our study, median post-shunt pressure reduction was 10.16 mm Hg, and at follow-up of 32 months, four patients developed shunt thrombosis, and two had rebleed. In the current era, percutaneous and transvenous mesocaval shunts were performed in selected cases with favourable anatomical factors, but with high risk of peritoneal bleeding, bowel injury, and shunt thrombosis [11,12].
Barman et al. [13] reported 2 cases of interposition splenorenal shunt in 1981; the causes were inadequate splenic vein length due to previous surgery, and splenic vein fibrosis in a pancreatic bed. He concluded that because of the formation of thin neointima, the PTFE graft had long-term shunt patency in the venous shunt. In our study, a common indication for iPSRS was portal hypertensive vascular changes in the splenic vein. We previously reported on our own experience with the pathological abnormalities of the splenic vessel in portal hypertension. Macroscopically, 93% of patients had splenic vein wall thickening, while 23% had vein calcification; pathologically, nearly all patients had median hypertrophy, and 23% had intimal fibrosis [4]. One of our patients underwent iPSRS due to a rare renal vein anomaly that was previously described as a case report; he later experienced partial shunt thrombosis that was managed by balloon angioplasty and metallic stent insertion [14].
A significant challenge in shunt surgeries is conduit diameter; the small size of conduits increases the incidence of shunt thrombosis and anastomotic stenosis. Sarfeh et al. [15] showed that a vein diameter of 4–6 mm could effectively decompress the portal pressure and reduce variceal bleed, and 85% of the shunt was patent on long-term follow-up. The first jejunal vein and left epigastroepiploic vein with more than 4 mm were used for conduits to decompress the portal system. Ellis et al. [16] described makeshift shunt or lesser shunt between lesser tributaries of portal circulation and systemic veins like IVC, renal, or gonadal vessels. Makeshift shunt patients in their study showed less regression of varices, and a high risk for rebleed. Warren et al. [17] also found that out of 4 makeshift shunt patients, 3 had rebleed. In our study, one interposition JCS patient had shunt thrombosis, but did not rebleed. Left gastroepiploic vein renal shunt thrombosed in both cases, and varices did not regress in one patient and bled within two years.
Splenectomy and devascularisation will be available, if unconventional shunt surgeries are not possible.
All patients had a reversal of hypersplenism. Most published series of unconventional shunt surgeries were in cirrhotic patients with less atheroma, and hence less thrombosis; NCPH has more atheroma and more thrombosis risk. There are some limitations to generalizing our results to common practice, as it is a small-size study, with no comparison group. It is important to note that shunt surgeries were performed in selected patients when medical management/non-surgical procedures failed. Hence, the outcomes of shunt surgeries and non-surgical procedures were not comparable.
Unconventional shunt surgery is effective in patients unsuited for other shunts, especially PSRS, and it achieves the desired effects in a significant proportion of patients.
Go to : 
ACKNOWLEDGEMENTS
The authors thank all residents who maintained portal hypertension data over 10 years in the Department of Surgical Gastroenterology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
Go to : 
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: HSL, BP. Data curation: HSL, BP. Methodology: All authors. Writing - original draft: HSL. Writing - review & editing: BP, SG, KR.
Go to : 
REFERENCES
1. Sarin SK, Sachdev G, Nanda R. 1986; Follow-up of patients after variceal eradication. A comparison of patients with cirrhosis, noncirrhotic portal fibrosis, and extrahepatic obstruction. Ann Surg. 204:78–82. DOI: 10.1097/00000658-198607000-00011. PMID: 3729586. PMCID: PMC1251226.
2. Poddar U, Thapa BR, Rao KL, Singh K. 2008; Etiological spectrum of esophageal varices due to portal hypertension in Indian children: is it different from the West? J Gastroenterol Hepatol. 23:1354–1357. DOI: 10.1111/j.1440-1746.2007.05102.x. PMID: 17683492.
3. Prasad AS, Gupta S, Kohli V, Pande GK, Sahni P, Nundy S. 1994; Proximal splenorenal shunts for extrahepatic portal venous obstruction in children. Ann Surg. 219:193–196. DOI: 10.1097/00000658-199402000-00011. PMID: 8129490. PMCID: PMC1243121.
4. Gupta S, Pottakkat B, Verma SK, Kalayarasan R, Chandrasekar A S, Pillai AA. 2020; Pathological abnormalities in splenic vasculature in non-cirrhotic portal hypertension: its relevance in the management of portal hypertension. World J Gastrointest Surg. 12:1–8. DOI: 10.4240/wjgs.v12.i1.1. PMID: 31984119. PMCID: PMC6943091.
5. Linton RR, Jones CM, Volwiler W. 1947; Portal hypertension; the treatment by splenectomy and splenorenal anastomosis with preservation of the kidney. Surg Clin North Am. 27:1162–1170. DOI: 10.1016/S0039-6109(16)32244-7. PMID: 20266148.
6. Mazariegos GV, Reyes J. 1998; A technique for distal splenoadrenal shunting in pediatric portal hypertension. J Am Coll Surg. 187:634–636. DOI: 10.1016/S1072-7515(98)00244-0. PMID: 9849740.
7. Gu S, Chang S, Chu J, Xu M, Yan Z, Liu DC, et al. 2012; Spleno-adrenal shunt: a novel alternative for portosystemic decompression in children with portal vein cavernous transformation. J Pediatr Surg. 47:2189–2193. DOI: 10.1016/j.jpedsurg.2012.09.007. PMID: 23217874.
8. Gupta S, Srinivas GV, Chandrasekar AS, Kalayarasan R, Pottakkat B. 2019; Splenoadrenal shunt for noncirrhotic portal hypertension. Indian J Surg. 81:28–31. DOI: 10.1007/s12262-017-1706-z.
9. Drapanas T. 1972; Interposition mesocaval shunt for treatment of portal hypertension. Ann Surg. 176:435–448. DOI: 10.1097/00000658-197210000-00001. PMID: 4263236. PMCID: PMC1355426.
10. Drapanas T, LoCicero J 3rd, Dowling JB. 1975; Hemodynamics of the interposition mesocaval shunt. Ann Surg. 181:523–533. DOI: 10.1097/00000658-197505000-00004. PMID: 124159. PMCID: PMC1345527.
11. Nyman UR, Semba CP, Chang H, Hoffman C, Dake MD. 1996; Percutaneous creation of a mesocaval shunt. J Vasc Interv Radiol. 7:769–773. DOI: 10.1016/S1051-0443(96)70847-3. PMID: 8897349.
12. Moriarty JM, Kokabi N, Kee ST. 2012; Transvenous creation of a mesocaval shunt: report of use in the management of extrahepatic portal vein occlusion. J Vasc Interv Radiol. 23:565–567. DOI: 10.1016/j.jvir.2011.09.023. PMID: 22464719.
13. Barman AA, Batiuchok W, Chaudry SS, Creedon JJ. 1981; Use of interposed polytetrafluorethylene (PTFE) graft in distal splenorenal shunt. Cardiovasc Dis. 8:555–557. PMID: 15216183. PMCID: PMC288001.
14. Biju P, Midha K, Gupta S, Kalayarasan R, Gnanasekaran S. 2019; proximal splenorenal shunt in a rare renal vein anomaly: a case report. Cureus. 25:e4754. DOI: 10.7759/cureus.4754. PMID: 31363436. PMCID: PMC6663117.
15. Sarfeh IJ, Rypins EB, Conroy RM, Mason GR. 1983; Portacaval H-graft: relationships of shunt diameter, portal flow patterns and encephalopathy. Ann Surg. 197:422–426. DOI: 10.1097/00000658-198304000-00008. PMID: 6600914. PMCID: PMC1352755.
16. Ellis DS, Linton RR, Jones CM. 1956; Effect of venous-shunt surgery on liver function in patients with portal hypertension; a follow-up study of 125 patients operated on in the last ten years. N Engl J Med. 254:931–936. DOI: 10.1056/NEJM195605172542002. PMID: 13322188.
17. Warren WD, Henderson JM, Millikan WJ, Galambos JT, Bryan FC. 1988; Management of variceal bleeding in patients with noncirrhotic portal vein thrombosis. Ann Surg. 207:623–634. DOI: 10.1097/00000658-198805000-00017. PMID: 3259859. PMCID: PMC1493505.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download