Abstract
Helicobacter pylori is a gram-negative pathogen commonly associated with peptic ulcer disease and gastric cancer. H. pylori infection has also been reported in cholelithiasis, cholecystitis, gallbladder polyps, and biliary tract cancers. However, the association between H. pylori and gallbladder and biliary tract pathologies remains unclear due to the paucity of literature. In response to the current literature gap, we aim to review and provide an updated summary of the association between H. pylori with gallbladder and biliary tract diseases and its impact on their clinical management. Relevant peer-reviewed studies were retrieved from Medline, PubMed, Embase, and Cochrane databases. We found that H. pylori infection was associated with cholelithiasis, chronic cholecystitis, biliary tract cancer, primary sclerosing cholangitis, and primary biliary cholangitis but not with gallbladder polyps. While causal links have been reported, prospective longitudinal studies are required to conclude the association between H. pylori and gallbladder pathologies. Clinicians should be aware of the implications that H. pylori infection has on the management of these diseases.
Helicobacter pylori is a gram-negative, microaerophilic, helix-shaped bacterium that infects over half of the population worldwide [1]. H. pylori is linked to several gastrointestinal diseases, including chronic gastritis, duodenal ulcer, gastric adenocarcinoma, and non-Hodgkin's lymphoma of the stomach [2-5]. Various factors, including the ability to produce the urease enzyme, which facilitates alkalinization through the conversion of urea into ammonia, enable H. pylori to adapt to a hostile acidic gastric environment [6].
Evidence on the relationship between H. pylori infection of the gallbladder and biliary tract diseases remains unclear. In recent years, there has been accumulating data demonstrating the correlation of H. pylori with gallbladder pathologies like cholelithiasis, cholecystitis, choledocholithiasis, gallbladder polyps, and biliary tract cancer [7-9]. Other studies have also reported that chronic gallbladder inflammation due to H. pylori infection causes biliary cancer [9,10]. On the other hand, some studies have reported there is no association between H. pylori infection and gallbladder diseases [11,12]. Furthermore, it is reported that patients with gallstone diseases experience an overall increased mortality risk. This, together with the high prevalence of gallstones, makes it important to evaluate the role of H. pylori in gallbladder diseases and biliary tract cancers. This review aims to summarise the literature concerning the relationship between H. pylori in the gallbladder and the occurrence of gallbladder and biliary tract diseases.
Relevant studies were retrieved from Medline, PubMed, Embase, and Cochrane databases, with the last search being conducted in December 2021. A combination of search terms such as “Helicobacter pylori” or “H. pylori”, “gallstone disease”, “cholelithiasis”, “choledocholithiasis”, “cholangitis”, “gallbladder cancer”, “cholangiocarcinoma”, and “biliary tract cancer” was used. Manual retrieval of eligible studies from references mentioned in the review articles was also conducted. We will first draw the link between H. pylori and its relationship with the biliary system before delving into hepatobiliary condition-specific interactions with H. pylori.
For any infectious agent to cause an infection in the body, it must fulfill two fundamental principles—the ability to arrive at the said location and the ability to survive in that environment. Although predominantly a colonizer of the gastric mucosa, H. pylori has been detected in the biliary system, suggesting potential translocation and, by extension, the potential for pathogenicity. The two current prevailing theories suggest that H. pylori may enter bile either through retrograde reflux from the sphincter of Oddi or hematologically from the portal circulation [13,14].
After translocating to the biliary system, it is also important to consider how it can survive in a relatively alkaline environment. One possibility is that the reflux of bile from the duodenum into the stomach plays a role in selecting specific H. pylori strains that are resistant to bile salt [15]. Another possibility is that inflammation from biliary pathologies may inadvertently lower biliary pH leading to a more favorable environment for H. pylori [16]. This shows that H. pylori can reach and survive in the gallbladder. This provides both the means and the path for H. pylori, a known group 1 carcinogen to cause chronic inflammation and malignancy in the hepatobiliary system.
There are many techniques for detecting H. pylori, but no gold standard method exists [17]. The methods which directly demonstrate the presence of H. pylori in the biliary system include culture and histopathological examination of the gallbladder, bile culture, or culture of gallbladder mucosal scrapings. Indirect methods which confirm the presence of H. pylori include polymerase chain reaction (PCR) and serology. Depending on the method and type of sample used, detection rates vary. Table 1 [17-23] summarizes the advantages, disadvantages and accuracy of the current H. pylori detection techniques.
PCR has a high sensitivity in detecting H. pylori [24,25] by using primers of conserved genes. In addition, nested PCR (NPCR) has even higher specificity due to the amplification of a narrower sub-region [26]. This provides a much quicker and more specific way to detect the growth of H. pylori in samples as compared to histopathological analysis. However, the PCR method comes with typical flaws; it generates false-positive results due to non-specific primers [27,28]. NPCR further compounds the false positive rate because of its susceptibility to spray contamination [26,29].
Serological methods are relatively inexpensive, and most laboratories can perform them easily [30]. Furthermore, it is useful in situations where bacterial density is expected to be low [17]. However, serology-based diagnosis has been regarded as less reliable than testing gallbladder samples due to the cross-reactivity of antigens among the Helicobacter species themselves [31] as well as with Campylobacter organisms [32]. Furthermore, serology is not specific to H. pylori infection of the gallbladder as it only indicates the presence of H. pylori infection; and not necessarily within the gallbladder [33]. Thus, a clinical correlation of the symptoms present with the positive serology testing must be performed to determine if it is likely that H. pylori resulted in hepatobiliary infections.
Another method used to detect H. pylori in the gallbladder directly is histology. This is the most accurate and specific. The Giemsa stain is more routinely used among the different stains as it is simple and relatively inexpensive [23,34]. However, the sensitivity of histology is often affected by multiple factors, such as the site and pattern of colonization, previous antibiotic use, sample representativeness of the entire gallbladder, and pathology doctors’ diligence [17].
The H. pylori culture taken from bile or mucosal scrapings remains the definitive method for detecting the presence of H. pylori infection within the gallbladder. However, challenges arise in culturing viable H. pylori within the gallbladder. Studies that used frozen tissue to culture H. pylori have reported unsuccessful viable bacteria culturing from the gallbladder [35-37]. For example, in a study by Fox et al. [36], cultures taken from 46 subjects yielded no viable H. pylori, despite positive PCR results. This was attributed to the use of frozen specimens, which may have inadvertently undermined the viability of H. pylori. Furthermore, H. pylori is an oxygen-sensitive microaerophile that cannot survive under normal atmospheric oxygen tension. As such, this can further complicate and hinder the process of culturing and result in false negatives [38]. On the other hand, studies that directly inoculated tissue specimens onto a sterile culture medium could successfully culture H. pylori colonies from gallbladder mucosa [39,40]. This indicates that the presence of H. pylori DNA detected via PCR may not merely represent ‘dead’ material.
Given the present challenges to culture, it remains unknown whether H. pylori detection in the gallbladder, through other tests such as PCR and histology, represents an active invasion of the gallbladder or only enterohepatic circulation of the bacteria [33,41]. However, PCR technology remains promising and can become the gold standard in identifying H. pylori. There is also a need for better growth conditions for the culture of H. pylori from biliary samples as this would allow confirmation of the viability of H. pylori in the biliary system. Finally, microbial isolation via culture is inaccurate in patients treated with antibiotics based on a local antibiogram. For example, in a local audit of 262 acute cholangitis patients, only 95 patients (36.3%) had positive blood cultures [42].
Although less commonly known, H. pylori infection has been associated with cholelithiasis and cholecystitis. Various meta-analyses have examined the relationship between gallstones and H. pylori infection [43,44], and reported that patients with H. pylori infection of the gallbladder had a significantly higher risk of gallstones than the control group. Studies conducted by Zhang et al. [45] and Takahashi et al. [46] on the prevalence of gallstones following the eradication of H. pylori support this conclusion. In the study by Zhang et al. [45] involving 15,523 participants, authors reported that gallstone prevalence was significantly lower among H. pylori-eradicated patients compared with H. pylori-positive patients with no prior eradication (9.02% vs 9.47%; p < 0.0001). Takahashi et al. [46] found similar results with a sample size of 15,551 participants (6.08% vs 4.73%; p < 0.001). Thus, the possible role of H. pylori eradication in managing gallstone diseases should be investigated. Furthermore, based on the meta-analyses and studies that demonstrate a lower prevalence of gallstones in patients with prior H. pylori eradication, we can conclude that there is a possible link between H. pylori infection and gallstone disease. With H. pylori infection being easily treatable with antibiotics, it is important to recognize this association, especially in H. pylori endemic regions [47].
However, with the limitations of the current methods of diagnosis, it is difficult to determine if the H. pylori detected is truly from the gallbladder or the stomach. Neither urea breath tests nor serology can accurately detect H. pylori infection of the gallbladder. On the other hand, more specific detection methods, such as PCR require tissue samples and therefore are invasive. Consequently, they may not be practical unless the patient has a strong indication for cholecystectomy or invasive biliary tract procedures. We acknowledge that the reliability of these studies may be compromised by the method of detection.
In addition to being associated with a higher prevalence of gallstones, H. pylori infection can play a causal role in the pathogenesis of gallstones in three main ways. Firstly, H. pylori may act as a nidus for stone formation [38], providing a starting point for accumulating stones. Secondly, H. pylori infection of the gallbladder increases oxidative stress in the infected regions. Through the production of reactive oxygen species and reactive nitrogen species, which affects the absorptive and secretory function of the gallbladder, supersaturation of bile can occur, resulting in the formation of stones [39]. Lastly, H. pylori can increase the precipitation of calcium bilirubinate through its ability to produce urease. This enzyme increases the pH for calcium precipitation and induce enzymes that deconjugate bile [38]. However, these theories are limited by the inability to demonstrate active colonization of the gallbladder by H. pylori [39]. PCR remains the most used method for detecting H. pylori in gallbladder samples [48]. However it is unable to distinguish between live and dead bacteria, leading to the possibility of false-positive results [33]. Given that H. pylori can likely survive in the gallbladder, it is not unreasonable to suggest that this supports the possible presence of live H. pylori. However, most existing studies investigating the relationship between H. pylori infection and gallstones are cross-sectional and are therefore unable to establish a temporal relationship between gallstone formation and H. pylori infection [49].
To close the gaps in the existing literature, we suggest conducting prospective studies in two areas to investigate the causal relationship between H. pylori infection and gallstones. Firstly, to validate the results of the existing cross-sectional studies, we propose long-term follow-up studies of H. pylori-positive, H. pylori-negative, and H. pylori-eradicated patients. Secondly, prospective studies investigating the impact of eradicating H. pylori on gallstone prevalence and recurrence can be conducted. In summary, despite the difficulty of establishing causality at this time, future prospective studies and advancements in the detection methods may provide insight into the relationship between H. pylori infection and gallstones.
Determining the exact relationship between H. pylori infection and gallstones is important due to its implications on gallstone prevention and H. pylori eradication regimes. Given that both H. pylori infection and gallstones are common diseases, it is important to determine if eradicating H. pylori can prevent gallstones [47]. This may also inform the decision for prophylactic treatment in close contacts and routine screening for H. pylori infection. Furthermore, it is unclear whether existing regimens for eradicating H. pylori from the stomach are adequate for eradicating H. pylori from the biliary tract. With the increasing rate of antibiotic resistance to H. pylori infections, the optimum therapeutic regime for eradicating H. pylori from the gallbladder should be established to prevent further reductions in the efficacy of eradication therapies [47]. Therefore, it is crucial to establish the exact relationship between H. pylori infection and gallstones to determine if adjustment to existing treatment protocols is required.
There currently exists no proven association between H. pylori infection and gallbladder polyps. This could be attributed to the benign nature of gallbladder polyps which do not spark attention to its possible risk factors. Two retrospective case-control studies have investigated the relationship between H. pylori infection and gallbladder polyps, and reported conflicting results [8,50].
Xu et al. [8] reported a positive correlation between H. pylori infection and gallbladder polyps in a study including 17,971 participants. The H. pylori infection group had significantly higher incidence of gallbladder polyps than that of the control group (odd ratio = 1.160, p = 0.033). The formation of gallbladder polyps is widely believed to be due to an underlying chronic inflammatory process involving the gallbladder mucosa [50-52]. This study thus highlighted the possibility of H. pylori infection triggering a local inflammatory process and thereby contributing to an increased incidence of gallbladder polyps. On the contrary, in a study conducted by Zhang et al. [50] involving 5,107 participants, no significant correlation between H. pylori infection and gallbladder polyps was found (p = 0.110). While both studies used abdominal ultrasonography for diagnosis and considered the possible confounding effect of certain variables, such as age, sex, and body mass index, before data analysis, the method of accounting for the impact of such variables was done in two different ways (adjusted odds ratio vs. case-control matching). Due to the conflicting results of the two studies, no conclusion can be derived about the correlation between H. pylori infection and gallbladder polyps. Gallbladder polyps are an important risk factor for gallbladder cancer [53,54]. Further research, especially prospective studies, is important in clarifying whether H. pylori infection has a cause-and-effect relationship with gallbladder polyps and by extension, whether H. pylori eradication can help in its prevention.
H. pylori has been implicated in the pathogenesis of biliary tract cancers [55,56]. In a case-control study of 156 bile samples, Boonyanugomol et al. [10] detected a significantly greater prevalence of H. pylori in the bile samples of cholangiocarcinoma (CCA) patients (66.7%) as compared to cholelithiasis patients (41.5%) and the control group (25.0%) by PCR (p < 0.05 in both comparisons). Significantly more inflammatory changes at the portal zones were seen in CCA patients who tested positive for H. pylori, indicating a possible role of H. pylori in a preceding inflammatory process before the development of CCA. A case-control study by Hassan et al. [55] also compared the gallbladder mucosal histology of non-infected gallbladders to H. pylori-infected gallbladders and reported that there was a significant increase in mucosal hyperplasia (p = 0.028) as a well as metaplasia and dysplasia (p = 0.049) amongst H. pylori-infected gallbladders compared to non-infected gallbladders. These changes have been identified as precursor lesions of gallbladder cancers [57]. Based on these case-control studies, there is a positive association between H. pylori infection and biliary tract cancers.
Therefore, it is important to understand how H. pylori may result in biliary tract cancers as this will help to develop effective treatment. The ability of H. pylori to produce pro-oncogenic molecules such as CagA and VacA, in tandem with its promotion of a chronic inflammatory state, can result in an increased production of free radicals and the dysregulation of various proliferation pathways, like the nuclear factor kappa B (NF-κB) and JAK/STAT transcription pathway. Boonyanugomol et al. [10] had shown that the CagA gene was significantly higher in patients with CCA than with cholelithiasis (36.2% to 9.1%, p < 0.05), suggesting that it could be involved in the pathogenesis of CCA. CagA pathogenicity island is essential in the internalization of H. pylori in cholangiocytes [10]. This can lead to the activation and induction of various carcinogenic cascades. Moreover, an increased cell turnover through the H. pylori-induced inflammatory response can also result in an increased mutation rate. These chronic inflammatory processes predispose patients to the development of both gallbladder cancer and CCA [58,59].
A recent study by Wang et al. [60] identified H. pylori proteins with potential involvement in gallbladder cancer pathogenesis using a bioinformatics approach. Briefly, the UniProt database containing the entire H. pylori proteome was used to predict which H. pylori proteins may potentially target the nucleus of host cells. Through the localization of specific protein sequences called Nuclear Localisation Signal, it was possible to determine which of the 1,552 H. pylori proteins possessed nuclear targeting activity. Leveraging on this novel approach, future studies may employ similar techniques to identify proteins involved in the pathogenesis of other H. pylori implicated gallstone diseases. Furthermore, this opens new avenues for targeted therapy.
While these studies employed sensitive serological, PCR, and histopathological diagnostic methods, they are cross sectional, and thus a causal relationship between an H. pylori infection and biliary tract cancer could not be concretely determined. As a group, although biliary tract cancer are rare cancers, there is some evidence that it is rising [61]. Currently, surgery is the only chance of cure for biliary tract cancer, and there is a high recurrence rate even with adjuvant chemotherapy [62]. Given the poor prognosis of biliary tract cancer, further clarification on whether the eradication of H. pylori will reduce its prevalence is important [63]. The recommended test for H. pylori infection is a urea breath test [64]. While a significant proportion of patients who had a positive urea breath test also tested positive for H. pylori in their bile or gallbladder tissue by PCR, there is no evidence that a urea breath test would be able to prove whether H. pylori has been eradicated from the biliary tree [65]. Many such studies exclude patients with prior H. pylori treatment. Still, we believe that various non-invasive and invasive H. pylori tests could be compared in this group of patients to investigate their utility for a test of cure of H. pylori infection in the biliary tree.
It has been postulated that an infectious etiology such as H. pylori can result in primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) [66,67]. Nilsson et al. [25] reported a significant association between H. pylori infection with PSC and PBC. In a histological analysis of liver biopsies, 20/24 had PCR positivity for the Helicobacter genus, compared to 1/23 (p < 0.001). In this group of 20, 5 of 9 of the PSC patients and 4 of 11 of the PBC patients tested positive for H. pylori specific primers. Conversely, Boomkens et al. [68] did not detect any differences in the prevalence of PSC or PBC between the H. pylori positive and the control group (p = 0.783). While Nilsson’s control group included biopsies of healthy cadaveric livers, Boomkens’ results may have been less reliable as the choice of control may be a possible confounding factor as hepatitis B cirrhosis is associated with a concurrent H. pylori infection [69].
The etiology of PSC and PBC is elusive and with limited effective management strategies, outcomes are sub-optimal and this is an area of research interest [70]. We suggest conducting prospective studies on the causal relationship between H. pylori infections and PSC and PBC and long-term follow-up studies to study the effects of H. pylori eradication on the prognosis and outcomes of PSC and PBC (Fig. 1).
A ‘test and treat’ strategy can be adopted, especially in endemic regions for younger patients presenting with dyspepsia without red flags [64]. Besides relieving dyspepsia, reducing the risk of gastroduodenal ulcers, gastritis, and gastric cancer, and as the first-line treatment of low-grade gastric marginal zone mucosa-associated lymphoid tissue lymphoma [86,87], this strategy can also prevent biliary pathologies including cholecystitis, gallbladder polyps, and biliary tract cancers. Meanwhile, longitudinal follow-up studies can be conducted on these patients to support the hypothesis that H. pylori is involved in the pathogenesis of biliary pathologies. Locally, the implications of H. pylori on health remain poorly understood by the general population. By increasing public awareness and health literacy about this topic, screening within high-risk groups can be encouraged, and early treatment for patients in need can be achieved [88]. Furthermore, a recent meta-analysis also shows that technology enhanced communication strategies improve compliance and eradication rates [89].
H. pylori infection is associated with cholelithiasis, chronic cholecystitis, biliary tract cancer, PSC, and PBC but not with gallbladder polyps. However, prospective longitudinal studies with longer follow-ups are needed to confirm the causal links. Nevertheless, as H. pylori is a common infection globally, clinicians should be aware of these associations. We anticipate that with emerging data, the implications of H. pylori on the prevention, screening, and management of patients with gallbladder pathologies will be confirmed.
REFERENCES
1. Lehours P. 2018; Actual diagnosis of Helicobacter pylori infection. Minerva Gastroenterol Dietol. 64:267–279. DOI: 10.23736/S1121-421X.18.02494-7. PMID: 29652111.
2. Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. 1991; Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 325:1127–1131. DOI: 10.1056/NEJM199110173251603. PMID: 1891020.
3. Veldhuyzen van Zanten SJ, Sherman PM. 1994; Helicobacter pylori infection as a cause of gastritis, duodenal ulcer, gastric cancer and nonulcer dyspepsia: a systematic overview. CMAJ. 150:177–185. PMID: 8287340. PMCID: PMC1486230.
4. Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, et al. 1994; Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 330:1267–1271. DOI: 10.1056/NEJM199405053301803. PMID: 8145781.
5. Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. 2001; Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 345:784–789. DOI: 10.1056/NEJMoa001999. PMID: 11556297.
6. Celli JP, Turner BS, Afdhal NH, Keates S, Ghiran I, Kelly CP, et al. 2009; Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc Natl Acad Sci U S A. 106:14321–14326. DOI: 10.1073/pnas.0903438106. PMID: 19706518. PMCID: PMC2732822.
7. Popescu D, Andronescu D, Babes PA. 2018; The association between Helicobacter pylori infection and liver and biliary tract disorders. Curr Health Sci J. 44:186–191. DOI: 10.12865/CHSJ.44.02.16. PMID: 30687530. PMCID: PMC6320469.
8. Xu MY, Ma JH, Yuan BS, Yin J, Liu L, Lu QB. 2018; Association between Helicobacter pylori infection and gallbladder diseases: a retrospective study. J Gastroenterol Hepatol. 33:1207–1212. DOI: 10.1111/jgh.14054. PMID: 29178198.
9. Avilés-Jiménez F, Guitron A, Segura-López F, Méndez-Tenorio A, Iwai S, Hernández-Guerrero A, et al. 2016; Microbiota studies in the bile duct strongly suggest a role for Helicobacter pylori in extrahepatic cholangiocarcinoma. Clin Microbiol Infect. 22:178.e11–178.e22. DOI: 10.1016/j.cmi.2015.10.008. PMID: 26493848.
10. Boonyanugomol W, Chomvarin C, Sripa B, Bhudhisawasdi V, Khuntikeo N, Hahnvajanawong C, et al. 2012; Helicobacter pylori in Thai patients with cholangiocarcinoma and its association with biliary inflammation and proliferation. HPB (Oxford). 14:177–184. DOI: 10.1111/j.1477-2574.2011.00423.x. PMID: 22321036. PMCID: PMC3371200.
11. Fallone CA, Tran S, Semret M, Discepola F, Behr M, Barkun AN. 2003; Helicobacter DNA in bile: correlation with hepato-biliary diseases. Aliment Pharmacol Ther. 17:453–458. DOI: 10.1046/j.1365-2036.2003.01424.x. PMID: 12562460.
12. Patnayak R, Reddy V, Jena A, Gavini S, Thota A, Nandyala R, et al. 2016; Helicobacter pylori in cholecystectomy specimens-morphological and immunohistochemical assessment. J Clin Diagn Res. 10:EC01–EC03. DOI: 10.7860/JCDR/2016/14802.7716. PMID: 27437221. PMCID: PMC4948397.
13. Waluga M, Kukla M, Żorniak M, Bacik A, Kotulski R. 2015; From the stomach to other organs: Helicobacter pylori and the liver. World J Hepatol. 7:2136–2146. DOI: 10.4254/wjh.v7.i18.2136. PMID: 26328025. PMCID: PMC4550868.
14. Helaly GF, El-Ghazzawi EF, Kazem AH, Dowidar NL, Anwar MM, Attia NM. 2014; Detection of Helicobacter pylori infection in Egyptian patients with chronic calcular cholecystitis. Br J Biomed Sci. 71:13–18. DOI: 10.1080/09674845.2014.11669957. PMID: 24693570.
15. Caldwell MT, McDermott M, Jazrawi S, O'Dowd G, Byrne PJ, Walsh TN, et al. 1995; Helicobacter pylori infection increases following cholecystectomy. Ir J Med Sci. 164:52–55. DOI: 10.1007/BF02968117. PMID: 7890538.
16. Magnuson TH, Lillemoe KD, Zarkin BA, Pitt HA. 1992; Patients with uncomplicated cholelithiasis acidify bile normally. Dig Dis Sci. 37:1517–1522. DOI: 10.1007/BF01296496. PMID: 1395997.
17. Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G. 2014; Diagnosis of Helicobacter pylori: what should be the gold standard? World J Gastroenterol. 20:12847–12859. DOI: 10.3748/wjg.v20.i36.12847. PMID: 25278682. PMCID: PMC4177467.
18. Clayton C, Kleanthous K, Tabaqchali S. 1991; Detection and identification of Helicobacter pylori by the polymerase chain reaction. J Clin Pathol. 44:515–516. DOI: 10.1136/jcp.44.6.515. PMID: 2066432. PMCID: PMC496836.
19. De Reuse H, Labigne A, Mengin-Lecreulx D. 1997; The Helicobacter pylori ureC gene codes for a phosphoglucosamine mutase. J Bacteriol. 179:3488–3493. DOI: 10.1128/jb.179.11.3488-3493.1997. PMID: 9171391. PMCID: PMC179139.
20. Lee JW, Lee DH, Lee JI, Jeong S, Kwon KS, Kim HG, et al. 2010; Identification of Helicobacter pylori in gallstone, bile, and other hepatobiliary tissues of patients with cholecystitis. Gut Liver. 4:60–67. DOI: 10.5009/gnl.2010.4.1.60. PMID: 20479914. PMCID: PMC2871607.
21. Singh V, Mishra S, Rao GR, Jain AK, Dixit VK, Gulati AK, et al. 2008; Evaluation of nested PCR in detection of Helicobacter pylori targeting a highly conserved gene: HSP60. Helicobacter. 13:30–34. DOI: 10.1111/j.1523-5378.2008.00573.x. PMID: 18205663. PMCID: PMC2752369.
22. Mishra RR, Tewari M, Shukla HS. 2011; Helicobacter pylori and pathogenesis of gallbladder cancer. J Gastroenterol Hepatol. 26:260–266. DOI: 10.1111/j.1440-1746.2010.06435.x. PMID: 21261714.
23. Lee JY, Kim N. 2015; Diagnosis of Helicobacter pylori by invasive test: histology. Ann Transl Med. 3:10. DOI: 10.3978/j.issn.2305-5839.2014.11.03. PMID: 25705642. PMCID: PMC4293485.
24. Clayton CL, Kleanthous H, Coates PJ, Morgan DD, Tabaqchali S. 1992; Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol. 30:192–200. DOI: 10.1128/jcm.30.1.192-200.1992. PMID: 1734052. PMCID: PMC265019.
25. Nilsson HO, Taneera J, Castedal M, Glatz E, Olsson R, Wadström T. 2000; Identification of Helicobacter pylori and other Helicobacter species by PCR, hybridization, and partial DNA sequencing in human liver samples from patients with primary sclerosing cholangitis or primary biliary cirrhosis. J Clin Microbiol. 38:1072–1076. DOI: 10.1128/JCM.38.3.1072-1076.2000. PMID: 10698999. PMCID: PMC86342.
26. Yu G, Fadrosh D, Goedert JJ, Ravel J, Goldstein AM. 2015; Nested PCR biases in interpreting microbial community structure in 16S rRNA gene sequence datasets. PLoS One. 10:e0132253. DOI: 10.1371/journal.pone.0132253. PMID: 26196512. PMCID: PMC4509648.
27. Sugimoto M, Wu JY, Abudayyeh S, Hoffman J, Brahem H, Al-Khatib K, et al. 2009; Unreliability of results of PCR detection of Helicobacter pylori in clinical or environmental samples. J Clin Microbiol. 47:738–742. DOI: 10.1128/JCM.01563-08. PMID: 19129407. PMCID: PMC2650922.
28. Sulo P, Šipková B. 2021; DNA diagnostics for reliable and universal identification of Helicobacter pylori. World J Gastroenterol. 27:7100–7112. DOI: 10.3748/wjg.v27.i41.7100. PMID: 34887630. PMCID: PMC8613642.
29. Šeligová B, Lukáč Ľ, Bábelová M, Vávrová S, Sulo P. 2020; Diagnostic reliability of nested PCR depends on the primer design and threshold abundance of Helicobacter pylori in biopsy, stool, and saliva samples. Helicobacter. 25:e12680. DOI: 10.1111/hel.12680. PMID: 32057175.
30. Leong RW, Sung JJ. 2002; Review article: Helicobacter species and hepatobiliary diseases. Aliment Pharmacol Ther. 16:1037–1045. DOI: 10.1046/j.1365-2036.2002.01282.x. PMID: 12030944.
31. Shimoyama T, Takahashi R, Abe D, Mizuki I, Endo T, Fukuda S. 2010; Serological analysis of Helicobacter hepaticus infection in patients with biliary and pancreatic diseases. J Gastroenterol Hepatol. 25 Suppl 1:S86–S89. DOI: 10.1111/j.1440-1746.2010.06224.x. PMID: 20586873.
32. On SL. 1996; Identification methods for campylobacters, helicobacters, and related organisms. Clin Microbiol Rev. 9:405–422. DOI: 10.1128/CMR.9.3.405. PMID: 8809468. PMCID: PMC172901.
33. Shukla HS, Tewari M. 2012; Discovery of Helicobacter pylori in gallbladder. Indian J Gastroenterol. 31:55–56. DOI: 10.1007/s12664-012-0178-0. PMID: 22553121.
34. Rotimi O, Cairns A, Gray S, Moayyedi P, Dixon MF. 2000; Histological identification of Helicobacter pylori: comparison of staining methods. J Clin Pathol. 53:756–759. DOI: 10.1136/jcp.53.10.756. PMID: 11064668. PMCID: PMC1731087.
35. Chen W, Li D, Cannan RJ, Stubbs RS. 2003; Common presence of Helicobacter DNA in the gallbladder of patients with gallstone diseases and controls. Dig Liver Dis. 35:237–243. DOI: 10.1016/S1590-8658(03)00060-4. PMID: 12801034.
36. Fox JG, Dewhirst FE, Shen Z, Feng Y, Taylor NS, Paster BJ, et al. 1998; Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 114:755–763. DOI: 10.1016/S0016-5085(98)70589-X. PMID: 9516396.
37. Bostanoğlu E, Karahan ZC, Bostanoğlu A, Savaş B, Erden E, Kiyan M. 2010; Evaluation of the presence of Helicobacter species in the biliary system of Turkish patients with cholelithiasis. Turk J Gastroenterol. 21:421–427. DOI: 10.4318/tjg.2010.0130. PMID: 21331997.
38. Grigor'eva IN, Romanova TI. 2020; Gallstone disease and microbiome. Microorganisms. 8:835. DOI: 10.3390/microorganisms8060835. PMID: 32498344. PMCID: PMC7356158.
39. Zhou D, Guan WB, Wang JD, Zhang Y, Gong W, Quan ZW. 2013; A comparative study of clinicopathological features between chronic cholecystitis patients with and without Helicobacter pylori infection in gallbladder mucosa. PLoS One. 8:e70265. DOI: 10.1371/journal.pone.0070265. PMID: 23936177. PMCID: PMC3728185.
40. Abayli B, Colakoglu S, Serin M, Erdogan S, Isiksal YF, Tuncer I, et al. 2005; Helicobacter pylori in the etiology of cholesterol gallstones. J Clin Gastroenterol. 39:134–137. PMID: 15681909.
41. Pellicano R, Ménard A, Rizzetto M, Mégraud F. 2008; Helicobacter species and liver diseases: association or causation? Lancet Infect Dis. 8:254–260. DOI: 10.1016/S1473-3099(08)70066-5. PMID: 18353266.
42. Mohan R, Wei Lynn Goh S, Tan GW, Tan YP, Junnarkar SP, Huey CWT, et al. 2021; Validation of Tokyo Guidelines 2007 and Tokyo Guidelines 2013/2018 criteria for acute cholangitis and predictors of in-hospital mortality. Visc Med. 37:434–442. DOI: 10.1159/000516424. PMID: 34722727. PMCID: PMC8543341.
43. Wang L, Chen J, Jiang W, Cen L, Pan J, Yu C, et al. 2021; The relationship between Helicobacter pylori infection of the gallbladder and chronic cholecystitis and cholelithiasis: a systematic review and meta-analysis. Can J Gastroenterol Hepatol. 2021:8886085. DOI: 10.1155/2021/8886085. PMID: 33505946. PMCID: PMC7806380.
44. Zhou D, Zhang Y, Gong W, Mohamed SO, Ogbomo H, Wang X, et al. 2011; Are Helicobacter pylori and other Helicobacter species infection associated with human biliary lithiasis? A meta-analysis. PLoS One. 6:e27390. DOI: 10.1371/journal.pone.0027390. PMID: 22087306. PMCID: PMC3210793.
45. Zhang FM, Yu CH, Chen HT, Shen Z, Hu FL, Yuan XP, et al. 2015; Helicobacter pylori infection is associated with gallstones: epidemiological survey in China. World J Gastroenterol. 21:8912–8919. DOI: 10.3748/wjg.v21.i29.8912. PMID: 26269681. PMCID: PMC4528034.
46. Takahashi Y, Yamamichi N, Shimamoto T, Mochizuki S, Fujishiro M, Takeuchi C, et al. 2014; Helicobacter pylori infection is positively associated with gallstones: a large-scale cross-sectional study in Japan. J Gastroenterol. 49:882–889. DOI: 10.1007/s00535-013-0832-z. PMID: 23736795.
47. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. 2017; Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 153:420–429. DOI: 10.1053/j.gastro.2017.04.022. PMID: 28456631.
48. Cen L, Pan J, Zhou B, Yu C, Li Y, Chen W, et al. 2018; Helicobacter pylori infection of the gallbladder and the risk of chronic cholecystitis and cholelithiasis: a systematic review and meta-analysis. Helicobacter. 23:e12457. DOI: 10.1111/hel.12457. PMID: 29266548.
49. Song JW, Chung KC. 2010; Observational studies: cohort and case-control studies. Plast Reconstr Surg. 126:2234–2242. DOI: 10.1097/PRS.0b013e3181f44abc. PMID: 20697313. PMCID: PMC2998589.
50. Zhang J, Zhang Y, Chen Y, Chen W, Xu H, Sun W. 2020; Helicobacter pylori is not a contributing factor in gallbladder polyps or gallstones: a case-control matching study of Chinese individuals. J Int Med Res. 48:300060520959220. DOI: 10.1177/0300060520959220. PMID: 33045881. PMCID: PMC7557694.
51. Andrén-Sandberg A. 2012; Diagnosis and management of gallbladder polyps. N Am J Med Sci. 4:203–211. DOI: 10.4103/1947-2714.95897. PMID: 22655278. PMCID: PMC3359430.
52. Kim KH. 2021; Gallbladder polyps: evolving approach to the diagnosis and management. Yeungnam Univ J Med. 38:1–9. DOI: 10.12701/yujm.2020.00213. PMID: 33045805. PMCID: PMC7787897.
53. Park JY, Hong SP, Kim YJ, Kim HJ, Kim HM, Cho JH, et al. 2009; Long-term follow up of gallbladder polyps. J Gastroenterol Hepatol. 24:219–222. DOI: 10.1111/j.1440-1746.2008.05689.x. PMID: 19054258.
54. Cairns V, Neal CP, Dennison AR, Garcea G. 2012; Risk and cost-effectiveness of surveillance followed by cholecystectomy for gallbladder polyps. Arch Surg. 147:1078–1083. DOI: 10.1001/archsurg.2012.1948. PMID: 22911224.
55. Hassan EH, Gerges SS, El-Atrebi KA, El-Bassyouni HT. 2015; The role of H. pylori infection in gall bladder cancer: clinicopathological study. Tumour Biol. 36:7093–7098. DOI: 10.1007/s13277-015-3444-9. PMID: 25877756.
56. Chang JS, Tsai CR, Chen LT. 2013; Medical risk factors associated with cholangiocarcinoma in Taiwan: a population-based case-control study. PLoS One. 8:e69981. DOI: 10.1371/journal.pone.0069981. PMID: 23894567. PMCID: PMC3718690.
57. Albores-Saavedra J, Alcántra-Vazquez A, Cruz-Ortiz H, Herrera-Goepfert R. 1980; The precursor lesions of invasive gallbladder carcinoma. Hyperplasia, atypical hyperplasia and carcinoma in situ. Cancer. 45:919–927. DOI: 10.1002/1097-0142(19800301)45:5<919::AID-CNCR2820450514>3.0.CO;2-4. PMID: 7260842.
58. Mishra SK, Kumari N, Krishnani N. Molecular pathogenesis of gallbladder cancer: an update. Mutat Res. 2019; 816-818:111674. DOI: 10.1016/j.mrfmmm.2019.111674. PMID: 31330366.
59. Labib PL, Goodchild G, Pereira SP. 2019; Molecular pathogenesis of cholangiocarcinoma. BMC Cancer. 19:185. DOI: 10.1186/s12885-019-5391-0. PMID: 30819129. PMCID: PMC6394015.
60. Wang Y, Imran A, Shami A, Chaudhary AA, Khan S. 2021; Decipher the Helicobacter pylori protein targeting in the nucleus of host cell and their implications in gallbladder cancer: an insilico approach. J Cancer. 12:7214–7222. DOI: 10.7150/jca.63517. PMID: 34729122. PMCID: PMC8558644.
61. Bridgewater JA, Goodman KA, Kalyan A, Mulcahy MF. 2016; Biliary tract cancer: epidemiology, radiotherapy, and molecular profiling. Am Soc Clin Oncol Educ Book. 35:e194–e203. DOI: 10.1200/EDBK_160831. PMID: 27249723.
62. Ghidini M, Tomasello G, Botticelli A, Barni S, Zabbialini G, Seghezzi S, et al. 2017; Adjuvant chemotherapy for resected biliary tract cancers: a systematic review and meta-analysis. HPB (Oxford). 19:741–748. DOI: 10.1016/j.hpb.2017.05.010. PMID: 28684194.
63. Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, et al. 2019; Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 37:1015–1027. DOI: 10.1200/JCO.18.02178. PMID: 30856044.
64. Chew CA, Lye TF, Ang D, Ang TL. 2017; The diagnosis and management of H. pylori infection in Singapore. Singapore Med J. 58:234–240. DOI: 10.11622/smedj.2017037. PMID: 28536725. PMCID: PMC5435840.
65. Bansal VK, Misra MC, Chaubal G, Datta Gupta S, Das B, Ahuja V, et al. 2012; Helicobacter pylori in gallbladder mucosa in patients with gallbladder disease. Indian J Gastroenterol. 31:57–60. DOI: 10.1007/s12664-012-0162-8. PMID: 22350820.
66. Terjung B, Spengler U. 2009; Atypical p-ANCA in PSC and AIH: a hint toward a "leaky gut"? Clin Rev Allergy Immunol. 36:40–51. DOI: 10.1007/s12016-008-8088-8. PMID: 18626795.
67. Lleo A, Leung PSC, Hirschfield GM, Gershwin EM. 2020; The pathogenesis of primary biliary cholangitis: a comprehensive review. Semin Liver Dis. 40:34–48. DOI: 10.1055/s-0039-1697617. PMID: 31537031.
68. Boomkens SY, de Rave S, Pot RG, Egberink HF, Penning LC, Rothuizen J, et al. 2005; The role of Helicobacter spp. in the pathogenesis of primary biliary cirrhosis and primary sclerosing cholangitis. FEMS Immunol Med Microbiol. 44:221–225. DOI: 10.1016/j.femsim.2004.11.002. PMID: 15866219.
69. Wang J, Chen RC, Zheng YX, Zhao SS, Li N, Zhou RR, et al. 2016; Helicobacter pylori infection may increase the risk of progression of chronic hepatitis B disease among the Chinese population: a meta-analysis. Int J Infect Dis. 50:30–37. DOI: 10.1016/j.ijid.2016.07.014. PMID: 27457918.
70. Marchioni Beery RM, Vaziri H, Forouhar F. 2014; Primary biliary cirrhosis and primary sclerosing cholangitis: a review featuring a women's health perspective. J Clin Transl Hepatol. 2:266–284. Erratum. DOI: 10.14218/JCTH.2015.00101. PMID: 26357630. PMCID: PMC4521232.
71. Eslami B, Iranshahi M, Gachkar L, Hadavand F. 2021; Gallstone frequency in patients with Helicobacter pylori gastritis. Arch Clin Infect Dis. 16:e100805. DOI: 10.5812/archcid.100805.
72. Kucuk S, Kucuk IG. 2021; The relationship between Helicobacter pylori and gallbladder pathologies, dysplasia and gallbladder cancer. Acta Med Mediterr. 37:2613–2620.
73. Mahmood A, Khan Z, Razzaq S, Mahmood MA, Ahmed N, Iqbal W. 2020; The likelihood of Helicobacter pylori presence in pre cholecystectomy gall bladder with or without cholecystitis - a case control study. Pak J Med Health Sci. 14:304–306.
74. Makkar R, Butt J, Huang WY, McGlynn KA, Koshiol J, Pawlita M, et al. 2020; Seropositivity for Helicobacter pylori and hepatobiliary cancers in the PLCO study. Br J Cancer. 123:909–911. DOI: 10.1038/s41416-020-0961-0. PMID: 32595210. PMCID: PMC7493958.
75. Kerawala A, Bakhtiar N, Abidi S, Awan S. 2019; Association of gallstone and Helicobacter pylori. J Med Sci. 27:269–272.
76. Ari A, Tatar C, Yarikkaya E. 2019; Relationship between Helicobacter pylori-positivity in the gallbladder and stomach and effect on gallbladder pathologies. J Int Med Res. 47:4904–4910. DOI: 10.1177/0300060519847345. PMID: 31434515. PMCID: PMC6833382.
77. Cherif S, Rais H, Hakmaoui A, Sellami S, Elantri S, Amine A. 2019; Linking Helicobacter pylori with gallbladder and biliary tract cancer in Moroccan population using clinical and pathological profiles. Bioinformation. 15:735–743. DOI: 10.6026/97320630015735. PMID: 31831956. PMCID: PMC6900325.
78. Fatemi SM, Doosti A, Shokri D, Ghorbani-Dalini S, Molazadeh M, Tavakoli H, et al. 2018; Is there a correlation between Helicobacter pylori and enterohepatic helicobacter species and gallstone cholecystitis? Middle East J Dig Dis. 10:24–30. DOI: 10.15171/mejdd.2017.86. PMID: 29682244. PMCID: PMC5903923.
79. Dar MY, Ali S, Raina AH, Raina MA, Shah OJ, Shah MA, et al. 2016; Association of Helicobacter pylori with hepatobiliary stone disease, a prospective case control study. Indian J Gastroenterol. 35:343–346. DOI: 10.1007/s12664-016-0675-7. PMID: 27633033.
80. Guraya SY, Ahmad AA, El-Ageery SM, Hemeg HA, Ozbak HA, Yousef K, et al. 2015; The correlation of Helicobacter pylori with the development of cholelithiasis and cholecystitis: the results of a prospective clinical study in Saudi Arabia. Eur Rev Med Pharmacol Sci. 19:3873–3880. PMID: 26531273.
81. Attaallah W, Yener N, Ugurlu MU, Manukyan M, Asmaz E, Aktan AO. 2013; Gallstones and concomitant gastric Helicobacter pylori infection. Gastroenterol Res Pract. 2013:643109. DOI: 10.1155/2013/643109. PMID: 23762037. PMCID: PMC3671525.
82. Abro AH, Haider IZ, Ahmad S. 2011; Helicobacter pylori infection in patients with calcular cholecystitis: a hospital based study. J Ayub Med Coll Abbottabad. 23:30–33. PMID: 22830140.
83. Yakoob J, Khan MR, Abbas Z, Jafri W, Azmi R, Ahmad Z, et al. 2011; Helicobacter pylori: association with gall bladder disorders in Pakistan. Br J Biomed Sci. 68:59–64. DOI: 10.1080/09674845.2011.11730324. PMID: 21706915.
84. Chen DF, Hu L, Yi P, Liu WW, Fang DC, Cao H. 2007; H pylori exist in the gallbladder mucosa of patients with chronic cholecystitis. World J Gastroenterol. 13:1608–1611. DOI: 10.3748/wjg.v13.i10.1608. PMID: 17461457. PMCID: PMC4146907.
85. Silva CP, Pereira-Lima JC, Oliveira AG, Guerra JB, Marques DL, Sarmanho L, et al. 2003; Association of the presence of Helicobacter in gallbladder tissue with cholelithiasis and cholecystitis. J Clin Microbiol. 41:5615–5618. DOI: 10.1128/JCM.41.12.5615-5618.2003. PMID: 14662950. PMCID: PMC309000.
86. Liou JM, Lee YC, El-Omar EM, Wu MS. 2019; Efficacy and long-term safety of H. pylori eradication for gastric cancer prevention. Cancers (Basel). 11:593. DOI: 10.3390/cancers11050593. PMID: 31035365. PMCID: PMC6562927.
87. Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, et al. 2012; Management of Helicobacter pylori infection--the Maastricht IV/Florence consensus report. Gut. 61:646–664. DOI: 10.1136/gutjnl-2012-302084. PMID: 22491499.
88. Teng TZJ, Sudharsan M, Yau JWK, Tan W, Shelat VG. 2021; Helicobacter pylori knowledge and perception among multi-ethnic Asians. Helicobacter. 26:e12794. DOI: 10.1111/hel.12794. PMCID: PMC7988653. PMID: 33656211.
89. Chua BQY, Chong VWS, Teng TZJ, Chia CTW, Aung MO, Shelat VG. 2022; Does technology-enhanced communication improve Helicobacter pylori eradication outcomes?-A meta-analysis. Helicobacter. 27:e12890. DOI: 10.1111/hel.12890. PMID: 35363943.
Fig. 1
Figure outlining the possible pathogenetic pathways of the various gallbladder diseases caused by Helicobacter pylori. CagA, cytotoxin-associated protein A; VacA, vacuolating cytotoxin A; IL, interleukin; ROS, reactive oxygen species; RNS, reactive nitrogen species; TNF-α, tumor necrosis factor alpha.
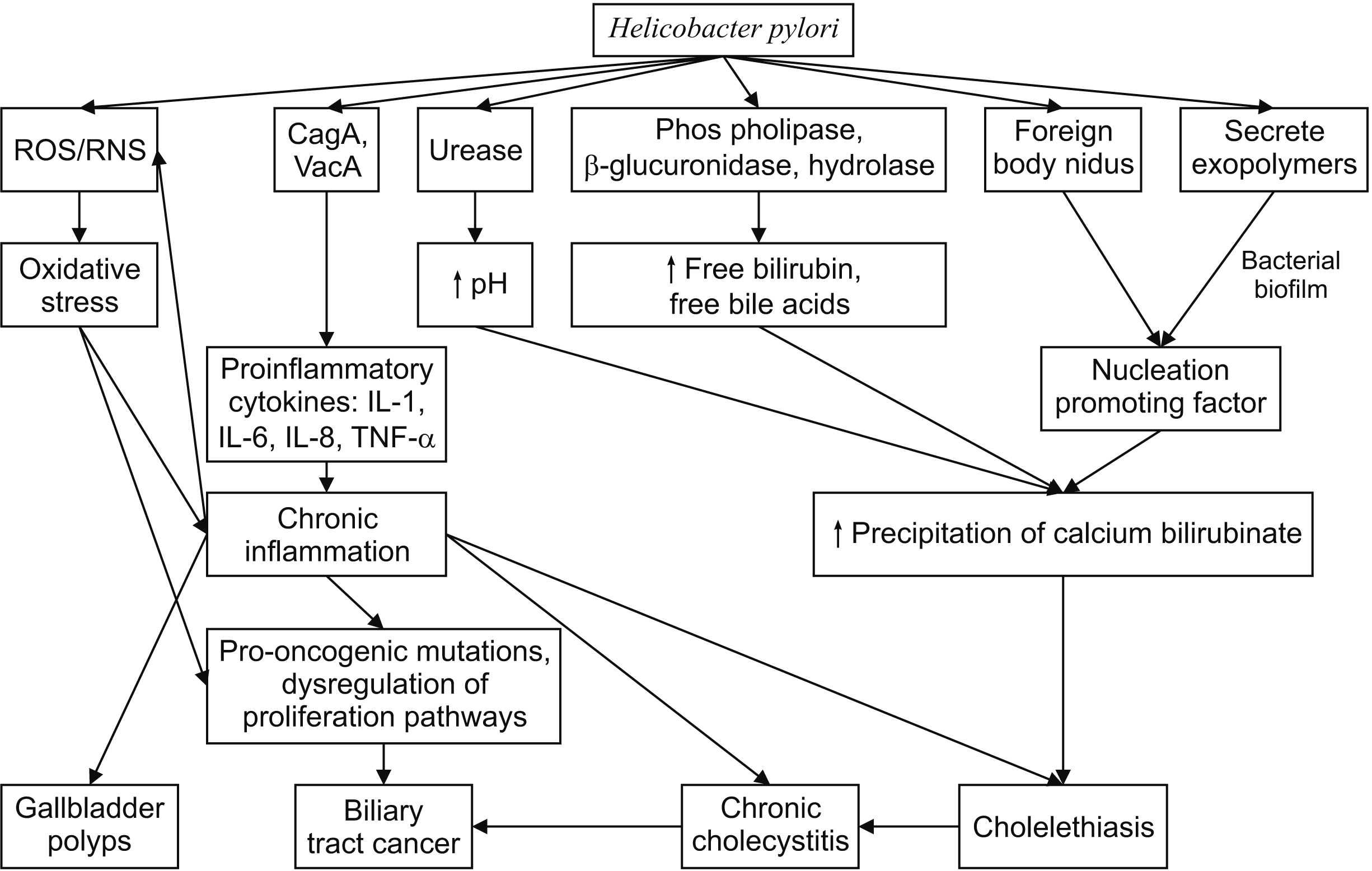
Table 1
Summary of Helicobacter pylori detection techniques
| Technique | Description | Advantage | Disadvantage | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Polymerase chain reaction | Primers of common conserved genes used to detect H. pylori are the urease A [18], urease C [19], 16S rRNA [20], Hsp60 gene [21] | High sensitivity and specificity | Susceptible to false positives | 63–100 [21] | 28–100 [21] |
| Serology | Utilizes enzyme-linked immunosorbent assay to detect serum H. pylori–specific immunoglobulin antibodies |
Inexpensive and easily performed Able to detect H. pylori even in cases with low bacterial density |
Cross reacts with antigens of other Helicobacter species and Campylobacter organisms Not specific for H. pylori infection of the gallbladder |
80– 90 [17] | 80– 90 [17] |
| Histology | Several stains used are the modified Giemsa, Warthin–Starry, Gimenez, Genta, and immunohistochemical H. pylori antibody stains | Able to directly demonstrate the presence of H. pylori in the gallbladder | Sensitivity is affected by factors such as site and pattern of colonization | 69– 93 [23] | 87–100 [23] |
| Microbial culture | Culture of H. pylori taken from bile or mucosal scrapings | Definitive method for demonstrating the presence of H. pylori infection in the gallbladder |
Hard to culture due to type of specimens used and fastidious nature of H. pylori virus Affected by prior antibiotic usage |
44 [22] | 67 [22] |
Table 2
Studies of Helicobacter pylori and biliary diseases
| Year | Reference | Condition | Method of diagnosis | Specimen | HP in subjects | HP in control | p-value |
|---|---|---|---|---|---|---|---|
| 2021 | Eslami et al. [71] | Cholelithiasis | H&E stain | Biopsy samples | 85 (50.9%) | - | 0.561 |
| Kucuk et al. [72] | Gallbladder cancer, chronic cholecystitis, cholelithiasis | Giemsa stain | Paraffin-embedded tissues | 68 (31.9%) | - |
0.010 (gallbladder cancer) 0.018 (chronic cholecystitis) |
|
| 2020 | Mahmood et al. [73] | Cholecystitis, cholelithiasis | PCR | Gallbladder tissues | 5 (11.4%) | 2 (0.06%) | 0.24 |
| Makkar et al. [74] | Biliary tract cancer, liver cancer | Serology | - | 74 | 357 | - | |
| Zhang et al. [50] | Polyps and cholelithiasis | Urea breath test | - | 12,735 (45.7%) | - | 0.110 | |
| 2019 | Kerawala et al. [75] | Cholelithiasis | Serology | - | 34 (75.6%) | 39 (86.7%) | 0.178 |
| Ari et al. [76] | Cholelithiasis | Giemsa stain | Stomach tissues, gallbladder tissues | 3 (11.1%) | 5 (15.2%) | 0.647 | |
| Cherif et al. [77] | Cholelithiasis, gallbladder cancer, biliary tract cancer | H&E stain | Biopsy samples | 48 (53.9%) | - |
< 0.001 (cholelithiasis) < 0.05 (cancer) |
|
| 2018 | Fatemi et al. [78] | Cholecystitis | PCR | Gallbladder tissues | 8 (15.4%) | 2 (3.8%) | 0.048 |
| Xu et al. [8] | Cholelithiasis, cholecystitis, gallbladder polyps | Serology | - | 7,803 (43.4%) | - |
0.101 (cholelithiasis) 0.012 (age-adjusted for cholelithiasis) 0.275 (cholecystitis) 0.033 (polyps) |
|
| 2016 | Dar et al. [79] | Choledocholithiasis | PCR | Bile samples | 20 (40.0%) | 0 (0%) | < 0.01 |
| 2015 | Hassan et al. [55] | Gallbladder cancer | Giemsa stain | Gallbladder tissues | 25 (50.0%) | - | 0.049 (metaplasia or dysplasia) |
| Guraya et al. [80] | Cholelithiasis | Serology | - | 75 (78.9%) | 12 (40.0%) | 0.001 | |
| Zhang et al. [45] | Cholelithiasis | Urea breath test | - | 3,410 (34.0%) | - | < 0.001 | |
| 2014 | Helaly et al. [14] | Cholecystitis | Immunohistochemistry | Gastric tissues, gallbladder tissues | 30 | - |
0.008 (gallbladder neck) 0.002 (gallbladder body) |
| Takahashi et al. [46] | Cholelithiasis | Serology | - | 15,551 | - | < 0.001 | |
| 2013 | Attaallah et al. [81] | Cholelithiasis | Rapid urease test, Giemsa stain, immunohistochemistry | Gastric tissues, gallbladder tissues |
Gastric: 47 (58.8%) Gallbladder: 21/94 (22.3%) |
- | 0.0001 |
| Zhou et al. [39] | Cholecystitis, Gallbladder cancer | WS stain | Gallbladder tissues | 64 (16.9%) | - | 0.022 (metaplasia) | |
| 2012 | Bansal et al. [65] | Cholelithiasis | Urea breath test, H&E stain, Giemsa & WS stain, PCR | Gallbladder tissues | 16 (32.7%) | 0 (0%) | 0.025 |
| 2011 | Abro et al. [82] | Cholecystitis | Serology, histopathology, rapid urease test, gram stain | Gallbladder tissues, bile | 55 (55.0%) | - | 0.03 |
| Yakoob et al. [83] | Cholelithiasis, cholecystitis, | H&E stain, WS stain, immunohistochemistry, PCR | Gallbladder tissues, bile | 22 (24.7%) | 5 (9.1%) | 0.02 | |
| 2007 | Chen et al. [84] | Cholecystitis | WS stain, PCR, immunohistochemistry | Gallbladder tissues | 35 (46.1%) | 16 (44.4%) | > 0.05 |
| 2005 | Boomkens et al. [68] | Primary biliary cirrhosis, primary sclerosing cholangitis | PCR | Liver tissue | 9 (29.0%) | 10 (34.5%) | Not significant |
| 2003 | Silva et al. [85] | Cholelithiasis, cholecystitis | Culture, PCR | Gallbladder tissues, bile |
Gallbladder tissue: 20 (31.3%) Bile: 24 (42.9%) |
- |
0.8 (cholelithiasis) 0.0003 (cholecystitis) |
| 2000 | Nilsson et al. [25] | Primary biliary cirrhosis, primary sclerosing cholangitis | PCR | Liver | 20 (83.3%) | 1 (4.3%) | < 0.00001 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download