Abstract
Hilar cholangiocarcinomas are highly aggressive malignancies. They are usually at an advanced stage at initial presentation. Surgical resection with negative margins is the standard of management. It provides the only chance of cure. Liver transplantation has increased the number of ‘curative’ procedures for cases previously considered to be unresectable. Meticulous and thorough preoperative planning is required to prevent fatal post-operative complications. Extended resection procedures, including hepatic trisectionectomy for Bismuth type IV tumors, hepatopancreaticoduodenectomy for tumors with extensive longitudinal spread, and combined vascular resection with reconstruction for tumors involving hepatic vascular structures, are challenging procedures with surgical indications expanded. Liver transplantation after the standardization of a neoadjuvant protocol described by the Mayo Clinic has increased the number of patients who can undergo operation.
Cholangiocarcinoma is a rare tumor of the bile duct epithelium that accounts for only 3% of gastrointestinal malignancies [1]. It has been divided into intrahepatic, hilar, and extrahepatic lesions, of which the hilar variety is the most common variant. Hilar cholangiocarcinoma (HC) was initially described by Altemeier et al. in 1957 [2], but was popularized by Klatskin in 1965 [3]. He described a peri-operative mortality of 92%, which has now been reduced to 8% by the advent of more sophisticated imaging procedures, an improvement of surgical techniques, and by preoperative interventions like biliary drainage to reduce the levels of jaundice and portal vein embolization (PVE) to cause hypertrophy of the remnant liver, if it is judged to be inadequate after a major resection is performed. However, even after 50 years operations for the tumor, its management remains a challenge for the surgeon.
The patients are typically elderly in their 6th decade of life with complaints of painless jaundice, abdominal pain, and weight loss [4]. Tumor markers, such as carcinoembryonic antigen and carbohydrate antigen 19-9 (CA19-9), can be used for screening, diagnosis, and monitoring, with a sensitivity and specificity of 89% and 86%, respectively. Imaging techniques, like ultrasound, computed tomography, and magnetic resonance cholangiopancreatography, help in assessing the resectability of the tumor (Fig. 1) [5].
Up to 65% of patients present with advanced or metastatic disease, which is associated with a poor prognosis, and a median survival of less than 1 year [6]. Complete R0 surgical resection is the only curative option, as the survival rates of R1 and R2 are low. Traditionally, the aim of surgical resection was the relief of jaundice, but over the last few decades, there has been a paradigm shift from local excision to cone excision of the liver at the hilum, and more extensive major liver resections, along with vascular reconstruction, which increase the survival rate [7]. Transplantation is the treatment modality for certain locally unresectable tumors, while palliative chemoradiotherapy (CRT) is the option for those that are widespread. In this article, we provide a brief overview of the available surgical options in the management of HC, discuss the controversies in the literature, and put forward possible recommendations.
The staging of HC first described by Bismuth et al. [8] in 1970, based on the level of bilary obstruction, is still the most common system. Although it is a morphological classification, it was widely used as a staging system. Bismuth et al. [8] divided HC into 4 types, where type 1 involves the extrahepatic duct, type 2 involves the hilum, type 3A the hilum with the right duct, 3B the hilum with the left duct, and type 4 the hilum, along with both right and left ductal systems. Though its efficacy has been proven, the limitation is its inability to predict the presence of distant metastases, lymph nodal and vascular involvement, and consequent lobar atrophy, and subsequently, patient survival.
Another staging system was proposed by Jarnagin et al. [6] from the Memorial Sloan Kettering Cancer Center, which system included local and tumor-related factors, like vascular involvement and lobar atrophy, irrespective of the presence of metastases or lymph nodal status. The modified T staging includes (i) biliary tract involvement, including the hilum and bilateral ducts, (ii) portal vein involvement, including contralateral infiltration, and (iii) the presence of lobar atrophy. This correlates with a resectability rate of 59% for T1 lesions and a median survival of 20 months, and 0% for T3 lesions, which have a median survival of only 8 months. However, this staging cannot be applied to patients who have lymph nodal and distant metastases.
The factors affecting the outcomes in patients with HC are the size of the primary lesion, the presence of metastases, the local extent of involvement, vascular invasion, the presence or absence of lobar atrophy, and the patient’s performance status. A new classification has been proposed by Blechacz et al. [9] from the Eastern Cooperative Oncology Group (ECOG), which classification includes tumor size, metastasis, and ECOG performance status. Deoliveira et al. [10] in 2011 described another staging system, which system uses tumor size, vascular involvement, and lymph node metastases. Using the criterion of size has its own limitations, because of our inability to predict this in the presence of local inflammation.
The TNM classification predicts the survival, but its limitation is that it is a pathological staging, and is only available after operation. This system incorporates the extent of tumor spread, biliary and vascular involvement, lymph node spread, and metastasis. The 7th edition of the American Joint Committee on Cancer (AJCC) was the first to provide different staging criteria for HC and distal cholangiocarcinoma. The 8th edition of the AJCC, which was introduced in 2018, has four main changes. Bilateral second-order bile duct involvement is no longer classified as T4; similarly, main portal vein involvement, previously classified as IIIb, has now been reclassified as IVa. Positive lymph nodes beyond the hepatoduodenal ligament have become M1 disease (stage IVb), rather than the N2 disease in the 7th edition. Instead, N2 disease (stage IVa) is now classified as 4 or more positive regional lymph nodes. Gaspersz et al. [11] have compared the prognostic accuracy of the 7th and 8th editions, and concluded that though the prognostic accuracy was similar in the majority, it was poor for patients who did not undergo resection. So, they recommended that cross-sectional imaging should be used to assess the staging, instead of only resection.
Though many staging systems are available for HC, none of them are perfect. While the Bismuth–Corlette, MSKCC, and TNM staging systems are the most commonly used, an improved system is still needed.
Advanced HCs require some form of liver resection, and the ability to perform safe major resections depends upon the necessary future liver remnant (FLR) to maintain the normal function of the liver. The quality of the FLR is affected by previous chemotherapy or any pre-existing liver disease, which in turn reduces the chances of a major resection. The mechanism of liver regeneration is complex involving an atrophy–hypertrophy complex when there is controlled liver regeneration, which occurs after hepatocyte injury by toxicity, occlusion, or trauma. The term hypertrophy is a misnomer, in that regeneration mainly occurs by hyperplasia i.e., increased number of cells, and partly by hypertrophy, i.e., increase in their individual size [12]. Usually, the liver has < 0.01% of cells undergoing mitosis, the rate of which increases depending upon the insult. Small insults result in localized regeneration, while insults involving more than 10% of the volume cause generalized regeneration, with 95% of cells undergoing mitosis. Various studies have shown that regeneration is stimulated by growth factors released by the liver, as well as from the extrahepatic organs. The portal vein plays a major role, as evidenced by the fact that the greatest regeneration occurs in the periportal zones, and the least near the hepatic veins [13]. The hypertrophy mechanism is multifactorial, with the hepatocyte growth factor playing an important role. Others are transforming growth factor-α and tumor necrosis factor, gut-derived growth factors, IL-6, nitrous oxide, and insulin [14].
An optimal FLR is not a constant figure, and the inability to achieve it is associated with a risk of post-hepatectomy liver failure, morbidity, and mortality. In the case of a normal liver, an FLR of at least 20% is considered optimal. If the patient has received previous chemotherapy for more than 12 weeks, the optimal FLR is 30%, while in patients with cirrhosis, it should be more than 40% [15]. Although various methods are available to augment the FLR, like PVE, Associating Liver Partition and Portal Vein Ligation with Staged hepatectomy (ALPPS), portal vein ligation, Assessment of Liver remnant volume using ICG clearance Intraoperatively during Vascular Exclusion (ALIIVE), and Associated Portal vein Embolization and Artery Ligation (APEAL), the first two are mostly used in HC [15].
The first report of PVE was published in 1990 by Makuuchi et al. [16], who found it induced the hypertrophy in the contralateral segment of the liver. Studies have indicated that regeneration following PVE is slower compared to resection, which causes necrosis rather than apoptosis following resection [17]. The traditional approach for PVE was by a mini-laparotomy under general anesthesia via the ileocolic vessels, but this has now been replaced by the percutaneous approach done under sedation, thus avoiding the risks of laparotomy and anesthesia. Percutaneous access can be ipsilateral or contralateral. The ipsilateral approach avoids damage to the FLR, but it is technically demanding, and requires advanced instruments, with the associated risks of tumor seeding. The contralateral approach is technically easier, but a potential risk for FLR damage exists. A short segment of the portal vein of about 1cm should be left for ligation during surgery. Various embolizing agents are available, like n-butyl cyanoacrylate and ethiodized oil, fibrin glue, ethanol, and microparticles, such as polyvinyl alcohol or trisacryl gelatin. While there are no randomized studies available comparing the agents, a review by Maundura and Koea [15] showed that in terms of an increase in the FLR, n-butyl cyanoacrylate was a better agent. The degree of liver hypertrophy depends upon the condition of the FLR. A normal liver has a hypertrophy rate of around 12−21 cm3/day (d) at 2 weeks, in comparison to 9 cm3/d in a cirrhotic liver. An adequate response is usually seen in 2−4 weeks in normal livers, while > 4 weeks is required in cirrhotic livers [14]. PVE is associated with a low complication rate and negligible mortality. In one meta-analysis consisting of 1,008 patients, the complication rate was 2.2%, and mortality 0%. Similarly, in another study by Di Stefano et al. [18], the complication rate was 6.4%, with no mortality.
Various controversies exist around PVE, like the timing of resection after the procedure, and the amount of post-resection regeneration and accompanying acceleration of tumor growth. The progression of the tumor following PVE creates confusion about the optimal waiting time for resection, which in turn is determined by the period required to attain the desired FLR. The average waiting period is 2−60 d [19]. Based on CT volumetry, a period of 3−4 weeks is considered adequate. The fact that the functional increase in the remnant is attained earlier than the increase in volume implies that there should be a shorter waiting period than that based on volumetric studies. Usually, there is a rapid increase in the FLR in the first 3 weeks, followed by a plateau phase, and then a slowing of growth. Thus, it is futile to wait beyond this time period. As per the experience of van Gulik et al. [20], CT volumetry is done after 21 d; if found inadequate, another waiting period of 14 d is optimum, and patients with an insufficient FLR are denied resection. Based on this, a waiting period of 2−4 weeks is recommended before a liver resection is performed.
Another area of controversy is tumor growth acceleration after PVE. Kokudo et al. [19] published a large series in which 19 patients who underwent resection post-PVE were compared with 29 who underwent resection without PVE, and found that the proliferation of metastatic deposits was higher in the PVE group. Tumor progression precluding the resection has been different in various studies. A meta-analysis showed an 11.3% unresectability rate due to progression post-PVE. Similarly, van Lienden et al. [21] showed a cancellation rate of 6.1%. The mechanisms involved in tumor growth post-PVE are changes in the cytokines and/or growth factors, like the upregulation of IL-6, TNF-α, and hepatocyte growth factors, alterations in the hepatic blood supply causing a compensatory increase in hepatic artery flow (the hepatic artery buffer response), and enhanced cellular host response promoting local tumor growth [20]. Hayashi et al. [22] found an acceleration of tumor growth of 0.59 cm3/d before PVE to 2.37 cm3/d after PVE with a median tumor growth acceleration ratio of 2.16 (range 1.00−7.46), indicating that the median tumor growth rate in the embolized lobe after PVE was about two times greater than that before PVE. The tumor growth rate was greater in hepatocellular carcinomas than HCs (2.65 vs. 1.16). Therefore, reducing the time interval between PVE and resection is recommended.
Patients requiring extended hepatectomies may require extended PVE with embolization of the segment IV branch, in addition to the standard PVE. This increases the FLR, and reduces the risk of liver failure. Nagino et al. [23] compared extended PVE against standard PVE, and found a greater increase in the FLR in the extended PVE group (51% vs. 30%). Similar findings were also shown by Kishi et al. (54% vs. 26%) [24]. The disadvantages of extended PVE are that it is (i) technically demanding, (ii) associated with a risk of embolization of the FLR in the case of migration of the agent used, (iii) injurious to the FLR, and (iv) accelerated tumor growth in the case of partial embolization [20].
Another dilemma is the exhaustion of the regenerative capacity of the liver post-PVE. However, various studies have found no difference in regeneration [25].
Recommendations: PVE is a good option to improve the FLR (Fig. 2). However, there are still many areas of controversies. Various embolizing agents are available for PVE, and n-butyl cyanoacrylate is probably best for improving the FLR. The optimal waiting period after PVE is 2−4 weeks, although this depends upon the degree of hypertrophy achieved. The risk of tumor growth acceleration is real with a chance of increasing unresectability, which can be minimized by reducing the waiting time. Extended PVE helps in augmentation of the real FLR with reduction in posthepatectomy complications, albeit it is technically complex (Level of recommendation II).
The first case of this technique was described in Germany in 2007 in a patient with HC. The surgeons were planning a right trisectionectomy, but intraoperatively found a small-volume left lateral segment. Hence, they spontaneously decided during surgery to try to quickly induce hypertrophy of the left lateral section by de-portalizing the right liver, while already having performed the parenchymal dissection along the right side of the falciform ligament, thereby completely devascularizing segment IV in preparation for the final resection to be performed in a second step. The concept was developed intra-operatively to make an otherwise non-resectable tumor resectable, in a novel 2-stage procedure. The approach was termed in situ split liver resection. It causes a rapid rise in the FLR by eliminating the intrahepatic, arterioportal, or portoportal collateral circulation, in addition to portal vein ligation. There was a 94% increase in the FLR after 8 d following the first ALPPS, which increased the enthusiasm among surgeons, but there was an associated high morbidity and mortality. Its proponents also believe that though it increases the FLR rapidly, and thus reduces drop-outs, tumors with an unfavorable biology will eventually progress, even after resection. The first publication from the international ALPPS registry showed a mortality of 27% in patients with HCs, but the overall mortality for various indications of ALPPS was 9% [26]. In another study, Olthof et al. [27] in 2017 compared ALPPS and PVE with right trisectionectomy for HC, and showed a high mortality (48% vs. 24%) and a low median survival (6 vs. 24 months) in the ALPPS group. In view of the associated morbidity, various authors have tried to modify the technique by preserving the middle hepatic vein during stage 1 [28], partial ALPPS [29], preserving the artery to segment IV [28], (all three techniques prevent segment IV necrosis), minimizing the skeletonization of the hilar structures, and abandoning the plastic bag that was used to wrap the transected liver [28]. The use of indocyanine green clearance to evaluate the liver function before proceeding to stage 2 has also been described, but that does not measure the function of the proposed FLR. A HIDA scan is the simplest and most effective method for functional evaluation of the FLR [30]. A number of variations have been proposed to simplify the procedure, such as: a) Central ALPPS: Here, segments IV, V, and VIII are preserved. A left lateral sectionectomy is performed in stage I, along with ligation of the right posterior portal vein, and an in situ split along the right posterior portal fissure [31]. b) No-touch ALPPS (hybrid ALPPS): This addresses the fundamental disadvantage of excessive tumor manipulation of the tumor in stage I. Here, the parenchymal transection is done via the anterior approach without dissection of the hilum, followed by PVE, while the second stage is performed 7−10 d later [32]. c) Associated Liver Tourniquet with Portal vein ligation for Staged hepatectomy (ALTPS): This was done to minimize the impact of stage I by making a groove along the plane of transection over the liver surface, followed by application of a Belghiti sling maneuver. The tourniquet is tightened till the portal vein flow into segment IV is occluded, as confirmed by intra-operative ultrasound [33]. d) Radiofrequency Assisted Liver Partition with Portal vein ligation (RALLP): This technique uses a series of radiofrequency coagulations along the proposed line of transaction to occlude the portal vein flow to segment IV. It is of particular help in laparoscopic surgery. The liver can then be easily transected with a knife in stage II along the line of coagulative necrosis [34]. e) Laparoscopic microwave ablation with portal vein ablation for staged hepatectomy (LAPS): This is identical to RALLP, except that it uses microwave energy, rather than radiofrequency energy [35].
Recommendation: PVE should be used as the initial method for volume augmentation, while ALPPS should be reserved for failed cases (Level of recommendation: II).
It is well-known that jaundice is associated with an increased risk of postoperative infection, renal failure, increased intraoperative blood loss, and post-operative liver failure. One of the causes of mortality of HC in the earlier eras was post-operative liver failure, which discouraged the surgeons from performing extensive resections. As shown in previous animal studies, cholestasis makes the liver more susceptible to ischaemia, reperfusion injury, and inflammation, by reducing its anti-oxidant ability and response to inflammation. The aim of preoperative biliary decompression (PBD) is to reduce the effects of cholestasis and promote liver regeneration, but it is not risk-free, but is associated with an increased risk of tumor seeding, extended hospital stays, morbidities, and infection [36].
The associated controversies are the optimum level of bilirubin to be attained, the duration of the drainage, and methods and types of drainage.
The optimal level of serum bilirubin has been different in various studies. Nimura et al. [37] and Makuuchi et al. [38] considered a cut-off of value of 3 mg/dL, while Hemming et al. preferred 5 mg/dL. Wiggers et al. [39] and Kennedy et al. [40] showed a reduction in postoperative failure rate and mortality following PBD, but this benefit could not be reproduced in FLRs of more than 30%, and the procedure should be avoided in patients with an FLR > 50% [36]. In cases of associated preoperative hypoalbuminaemia, anaemia, or renal dysfunction, PBD can help patients with a bilirubin level > 2.5 mg/dL, or alone with bilirubin > 6.5 mg/dL, as shown by Wronka et al [41]. A recent meta-analysis showed an unsuitability of PBD in HC patients, due to an increased risk of bile leakage, cholangitis, infection and intraoperative transfusion if the bilirubin was level < 15 mg/dL and a normal FLR, but this study has some limitations [36].
Recommendation: Though the optimal bilirubin level is still a matter of debate and various authors have recommended different targets, it is desirable to reduce the bilirubin < 5 mg/dL, before proceeding to major resections, to avoid post-hepatectomy liver failure (Level of recommendation: II).
The optimal duration of PBD is not clear, because of the risk of drain malfunction, inflammation surrounding the operative area with subsequent increased anastomotic leaks, and tumor progression in the case of longer waiting times. A prolonged PBD is also associated with low resection rates, due to migration of tumor cells via the fistula tract. Only two-thirds of patients achieve normal levels of bilirubin after PBD, and this depends upon the duration and level of preoperative hyperbilirubinaemia, so to wait for complete normalization is unwise. Previous studies have a waiting period varying 10−32 d, with complete normalization around 4−8 weeks, but Paik et al. [42] showed that a waiting period of more than 2 weeks was counterproductive.
Recommendation: The optimal waiting period after PBD is not clearly defined, and should be approached on a case-by-case basis; but depending upon available evidence, a waiting period of 2 weeks before surgery will probably suffice (Level of recommendation: II).
Regarding methods of biliary drainage in HC, there are 3 types: percutaneous transhepatic biliary drainage (PTBD), endoscopic retrograde biliary drainage (ERBD), and endoscopic nasobiliary drainage (ENBD), but there are no randomized trials available to compare them. ERBD is physiological, and causes an improvement in the patient’s nutritional status with a reduction in endotoxaemia and dyslipidaemia. Despite its advantages, it is technically challenging and complex, with a drawback of its inability to correctly identify the longitudinal extension of the tumor intraoperatively. The morbidity and mortality of endoscopic drainage is higher, in comparison to percutaneous drainage. PTBD is preferred over ERBD, in view of the low risk of cholangitis, and smaller number of procedures with more successful decompression. PTBD has the added advantage of obtaining a cholangiography with an exact definition of the extent of the tumor. The disadvantage is its invasiveness and risk of tumor seeding (5%−20%). As per various meta-analyses, ENBD is the initial method of choice for drainage, due to the associated lesser complication and inflammation around the bile duct, along with no risk of tumor spread. But it is not effective in type IV blocks, and there is also associated patient discomfort and self-removal (Fig. 3) [42].
Recommendation: ENBD should be considered for initial management of HCs, while PTBD should be reserved for advanced HCs, segmental cholangitis, and delayed resolution of jaundice (Level of recommendation: II).
It is important to rule out radiologically occult metastatic peritoneal carcinomatosis, which obviates the need for a formal exploration, and facilitates an early referral of patients for palliative chemotherapy (Fig. 4). Of all HCs, only 65% are found to be resectable at laparotomy [43]. Though this indicates the need for staging laparoscopy (SL), its role is still controversial in the literature, because HCs are usually locally advanced, and need formal exploration for resectability to be determined. Bird et al. [44] showed that out of 114 patients who underwent SL, only 31 patients were found to be unresectable with a yield of 27.2%, and the sensitivity for diagnosing peritoneal metastases was 71%. In another meta-analysis by Coelen et al. [45], the yield of SL had a wide range (6%−45%), with a pooled sensitivity of 24%. They also found the yield to be lower after 2010 because of better imaging modalities.
Recommendation: Current investigative modalities have reduced the yield of SL; however, it should be used to rule out peritoneal metastases, and prevent futile laparotomies (Level of recommendation: II).
The curative treatment for HC is complete resection. To achieve this, frozen section plays an important role. Though it has been used in various malignancies with considerable success; its role in HC is debatable, because of specific pathological features in HCs, like involvement along the longitudinal axis of the bile duct wall, and in the submucosal space with perineural, perilymphatic, and perivascular infiltration. The inability to further increase the already extensive resection performed after the clinical implication of a positive margin is also questionable. In a study of 67 patients by Mantel et al. [46], the sensitivity of FS was 68%, with a false negative rate of 16%. In their study, the contribution of FS to achieve an R0 secondary resection was low. Though various studies showed a secondary R0 resection of around 4%−9%, one study by Ribero et al. [47] showed a rate of 19%, but this was associated with a high risk of complications. A recent meta-analysis by Ke et al. [48] demonstrated comparable 5-year overall survivals between initial R0 and R0 after additional bile duct resection. They also found improved 5-year overall survival rates for patients with R0 after additional resection, in comparison to initial R1 (odds ratio [OR], 3.54; 95% confidence interval [CI], 1.67−7.50). In another retrospective study, frozen section benefitted 17 patients by classifying them to true R0. These 17 patients had an improved 5-year and median overall survival, without any difference in recurrence rates. A total of 7 patients could not undergo additional resection, due to anatomical reasons [49].
Recommendation: The aforementioned data supports the role of FS in HC, because it is linked to a better 5-year survival, depending upon the availability of a wide proximal resection tumor free margin (Level of recommendation: II).
The only curative option for HC is an R0 liver resection with an adequate FLR. The advent of better techniques and exposure to liver transplantation (LT) have paved the way for major hepatic resections with low morbidity and mortality from simple bypass procedures. The importance of an R0 resection was explained by DeOliveria et al. [50] in 2007 when they analyzed 564 (281 HC) patients with bile duct cancer operated during 1973−2004. R0 resections were performed in 52 patients, while R1 was performed in 121 patients. The median survivals (38 vs. 18 months) and 5-year survivals (27% vs. 7%) were better in the R0 group. After the confirmation of the beneficial effects of an R0 resection, various authors have divided the R0 group into those with wide margins R0 (> 5 mm), and narrow margins R0 (< 5 mm). Endo et al. [51] in their study found patients with wide margin R0 had a better survival than the narrow margin group, who had a similar survival to those with R1 resection. Seyama et al. [52] found a better survival in patients with a margin of more than 5 mm, in comparison to those with a margin of less than 5 mm. The survival was not statistically different between the narrow margin and R1 group. Sakamoto et al. [53] also confirmed the same by studying serial sections of 62 resected HC specimens, and found no anastomotic site recurrence in patients with wide margin resections [7].
Recommendation: A wide margin R0 resection provides a better chance of survival in HC patients. But the possibility of achieving wide margins is limited by the presence of the vascular structures at the liver hilum, and the inability to determine the exact microscopic tumor extension (Level of recommendation: II).
Traditionally, HCs were considered to be unresectable, and a simple bypass or bile duct intubation was usually performed to relieve the obstruction. With time, the curative intent procedures came into play from local excision to major hepatic resections. In the 1980s, surgeons considered HCs to be a regional disease rather than a local disease, and carried out an unnecessary number of liver resections without any major survival benefits, and the postoperative mortality rates increased from 9% in the case of local resections to 17% in major resections [7].But over the past two decades, with the improvement in patient selection, meticulous surgical techniques and the popularity of LT has led to major liver procedures being performed with low morbidity. Previously, the excision of the adjacent cone of liver parenchyma was considered to be adequate, but it is now judged to be inadequate.
Major hepatic resections have proven benefit in the type III and IV HCs, while their role in type I and II HCs is controversial. A few researchers believe that local resection with an adjacent cone of parenchyma is sufficient for I and II HCS [54,55]. However, Ikeyama et al. [56] recommended that major hepatic resection is required for the nodular and infiltrating types, while a limited resection will suffice for the papillary variety. Lim et al. [57] showed concomitant major liver resection is associated with low recurrence and better survival, with a similar post-operative morbidity and mortality.
Recommendation: Although the literature is inconclusive, parenchyma preserving hepatic resections like central hepatectomy in type I and II HCs is recommended (Level of recommendation: II).
The predominance of the side of the involvement determines the type of resection. In general, right hepatectomy is considered for type IIIa and type IV lesions with predominant right-side involvement, while left hepatectomy is preferred for type IIIb and type IV with predominant left-sided involvement. In centrally located tumors, which can be treated by either method, surgeons prefer a right hepatectomy due to various anatomical considerations, like the longer extrahepatic course of the left duct, the right-sided lie of the bile duct confluence, the right hepatic artery running behind the common duct with the risk of involvement by tumor, and anatomical variations being more on the right side, which may preclude a safe left hepatectomy. Various authors have compared both approaches, and found there was no difference in R0 resection rates, 5-year survival, and portal vein reconstruction rates, but it was associated with a significantly higher requirement for right hepatic artery reconstruction with a left hepatectomy [43]. Theoretically, right-sided resection allows for a greater margin, owing to the long extrahepatic course of the left duct, but the final pathological studies have shown little difference (14.5 vs. 13.7 mm) [58]. Although it is possible to obtain a wide resection margin in left hepatectomy by extending the dissection into the segmental ducts, the supraportal course of the right posterosectoral duct is the limiting factor. Nagino et al. [59] in their study of 574 patients performed more left-sided hepatectomies (51.8% vs. 38.3%), although type IV constituted the major proportion (45.5%) of their patients. Various authors have also found equal operative outcomes and long-term survivals in both forms of hepatectomies. Jo et al. [60], in their study of 83 patients, performed a right hepatectomy in 33 patients, and left hepatectomy in 24 patients. PVE was done in 18.2% of patients in the right hepatectomy group (0 in the left hepatectomy group). Posthepatectomy liver failure was seen in 21.2% in right-sided resections, in comparison to 8.3% in left-sided resections. However, the 5-year survival and recurrence free survival were similar in both groups.
Recommendation: Although the survival and recurrence rates for both types of resections are comparable, the right-sided resections are technically easier, and permit more vascular resections, but have a higher incidence of post-hepatectomy liver failure (Level of recommendation: II).
The rationale behind caudate lobe resection is that its duct drains near the hepatic confluence, which increases the risk of involvement by tumor, and its routine resection improves the rate of R0 resection. The importance of CL was shown by Nimura et al. [37] in 1990 in their case series, where they found 44 out of 45 patients showed involvement of branches of the caudate lobe. Bengmark et al. [61] performed major hepatic resections without CL, and R0 resection was achieved in only 18.2% cases. After this revelation, the rate of CL has increased worldwide. In an analysis of 580 patients with type III and IV blocks, 61% underwent CL; and when the CL resection was omitted, the rate of R1 resection was increased [43]. A recent meta-analysis showed an increased R0 resection (OR, 3.88; 95% CI, 2.18−6.90) and survival (hazard ratio, 0.45; 95% CI, 0.38−0.55), without any associated increase in morbidity and mortality [62].
Recommendation: Caudate lobe resection should be routinely employed in patients with HC (Level of recommendation: II).
The distal bile duct resection point is long and near the superior pancreatic margin, and usually ensures an R0 resection. However, in a few cases, it is not sufficiently long, and these patients require an extensive resection involving the liver, bile duct pancreatic head, and duodenum. The indications are diffusely infiltrating tumor of the extrahepatic bile duct, bulky nodal metastasis in the pancreaticoduodenal region in HC, and perihilar tumors showing downward superficial spread. Hepatopancreaticoduodenctomy (HPD), which was initially described for gall bladder cancer in 1980, has also been extended for HC. In the initial days, the role of HPD was questioned, due to the complications and poor survival. However, most of the recent reports indicate an improved survival and reduced mortality. Ebata et al. [63] performed 85 consecutive HPDs from 1992 to 2011, with an overall survival of 79.7%, and an operative mortality of 2.4%. Postoperative liver failure and postoperative pancreatic fistula were the most common and major complications. They concluded that although technically demanding and associated with high morbidity, HPD can be performed in selected patients, offering a better long-term survival.
Recommendation: In selected cases, and in centers with experience in performing liver, as well as pancreatic resections, HPD increases the number of resectable HCs, albeit with an increased number of complications (Level of recommendation: II).
The location of the hepatic artery and portal vein near the confluence of the bile ducts predisposes to their involvement by tumor. Traditionally, those with vascular involvement were considered to be unresectable, but with the improvement in microsurgical techniques and experience in LT, these tumors are now amenable to surgery with vascular reconstruction. Portal vein resection is now an established practice in HCs, with acceptable results shown by various high-volume centers. The ‘no touch’ technique described by Neuhaus et al. [64] is a form of en bloc resection, which includes resection of segments IV, V, VI, VII, and VIII with lymphadenectomy, and resection of the extrahepatic bile duct, portal vein bifurcation, and right hepatic artery avoiding the dissection around the tumor. They published their results in 2012, where they compared en bloc resections and major hepatectomies with 50 patients in each arm. They reported better 1-, 3-, and 5-year survivals in the en bloc resection group at 87%, 70%, and 58%, compared to major hepatectomy at 79%, 40%, and 29%, with comparable mortality. An international multi-institutional study by de Jong et al. [1] in 305 patients showed a higher 30-d (5% vs. 12%) and 90-d mortality (15% vs. 18%) in the portal vein resection group, although the results were not statistically significant. Wu et al. [65]’s analysis reported that mortality was lower in portal vein resections performed after 2007, in comparison with those done before 2007.
The role of concomitant hepatic artery resection and reconstruction is controversial, with a reported mortality of around 56%. The requirement of alternative liver inflow may be required sometimes via the gastroduodenal artery or left hepatic artery, or by the use of interposition grafts. Portal vein arterializations can also be used, but are associated with portal hypertension and biliary complications [66]. Various centers have reported poor results following hepatic artery resection, but the series by Nagino et al. [67] have shown better overall survival, and only 1 post-operative mortality.
Another en bloc resection approach is the Rex recess approach introduced by Rela et al. [68], and the portal vein reconstruction was achieved by an interposition graft. The Rex recess is the space between segments III and IV under the liver bridge. The resection line is away from the hilum, and further away from the area described by Neuhaus et al [64].
Recommendation: Portal vein resection helps to achieve better R0 resections with improved overall survival and acceptable complications, and should be offered to patients with portal vein invasion. Regarding hepatic artery resection, the role is still controversial, is associated with high morbidity and mortality, and should be performed in selected patients at experienced centers (Level of recommendation: II).
Lymph node involvement is an important prognostic marker in patients with HCs, and their involvement is facilitated by the thin bile duct wall. Lymphadenectomy in HCs is an important topic, as the incidence of metastasis is 31%−58%. This varies with the T stage as 0%, 36.7%, 23.8%, and 57.7% in T1, T2, T3, and T4, respectively, and Bismuth stage as 21.1%, 27.3%, 41.5%, and 55.6% in I, II, III, and IV, respectively [69]. As per the AJCC 8th edition, lymph node involvement outside the hepatoduodenal ligament is considered M1. In HCs, lymph node metastasis is common around the bile duct (27.1%−42.7%), followed by the portal vein (30.9%−35.7%), common hepatic artery (27.3%−31.3%), para-aortic region (17.3%), posterior pancreatic head (14.5%–50%), and coeliac trunk (6.4%−14.3%) [70]. As per the NCCN guidelines, standard lymph nodal clearance for HCs includes the lymph nodes along the hepatoduodenal ligament, while the posterior pancreatic head and metastasis beyond these areas is considered to be unresectable. However, a few authors like Kitagawa et al. [71] described extended lymphadenectomy as removal of the lymph nodes along the superior mesenteric vein and coeliac trunk. They showed the 3- and 5-year survival of patients with or without lymph node metastasis to be 31.8% and 14.6%, respectively, while for paraaortic lymph node metastasis, it was 12.3%. In the paraaortic lymph node positive group, 7 patients (out of 19) were diagnosed to have tumor involvement on final pathological examination, but were normal on gross appearance. The survival in these 7 patients was similar to patients with regional lymph node metastases, and better than in patients with intra-operative metastasis found in the para-aortic node. The 5-year survival rate of patients of HC with regional lymphadenectomy is 7%−20%, while in patients who underwent extended lymphadenectomy, it is 26%−49% [72]. Mantel et al. [73] found extended lymphadenectomy increased lymph node retrieval, and thus prevented under-staging and improved survival. But after propensity score matching, they found that extended lymphadenectomy improved survival in M0 patients with R0 resection, but had no effect in patients with M1 disease. They concluded that extended lymphadenectomy should not be adopted in patients with positive distant nodes.
Recommendation: Extended lymphadenectomy results in improved lymph node retrieval, and overall survival in M0 patients, but has no effect in patients with distant lymph node metastasis, and its routine employment in surgical practice requires further studies (Level of recommendation: II).
Minimally-invasive surgery has made great advances since its inception. It has improved short-term, without affecting long-term, oncological outcomes in liver resections, as shown in the OSLO−COMET trial [74]. However, because of the complex anatomy of the hilar region, along with its high incidence of anatomical variations, the adoption of minimally invasive surgery has been a little slow and guarded in HC operation. The earliest laparoscopic resection for HC was performed by Yu et al. [75] in 14 patients with Bismuth type I and II lesions. Due to the low R0 resection rates, and high mortality and morbidity in type II patients, they concluded that type I HCs could be resected laparoscopically, while in type II patients, the technique needed further validation. Many studies have been published following this report, but none included hemihepatectomy with caudate lobe resection. A report by Gumbs et al. [76] in 2012 included 5 HC patients, of whom 3 underwent concomitant laparoscopic right- or left-hepatectomy, with excellent post-operative recovery. Numerous studies were subsequently published, but all were in the form of case reports or case series, without any comments on their post-operative outcome in comparison with traditional open surgery. Zhang et al. [77] in 2019 compared laparoscopic radical resection with traditional open resection in terms of post-operative stay, blood loss, and complications, and found no significant difference between the approaches. However, the operative time was longer in the laparoscopic group with lower 1- and 2-year survival rates. While most studies have been from Asia, Ratti et al. [78] from Italy compared laparoscopy and open surgery in 2020. The comparable blood loss, short hospital stay, longer operative time and similar R0 rate were the short-term results of laparoscopy. Both groups had similar survival rates. He et al. [79] in 2022 compared 62 open to 21 laparoscopic surgeries for type III HCs, and concluded that laparoscopic resection was associated with less blood loss and longer operative times, with similar R0 rates, post-operative complications, and hospital stay.
The experience with robotic surgery in HC is also limited, owing to the lesser experience than with laparoscopic surgery. But the Endowrist within the da Vinci Surgical system helps in easy suturing and handling challenging maneuvers. Most of the studies for robotic resection are case reports or small case series. Xu et al. [80] in their series of 10 patients did not support the use of robotic surgery in HC, in view of the significantly longer operative time (703 vs. 475 min, p < 0.001), higher complication rate (90 vs. 50, p = 0.031), inferior recurrence-free survival, and higher hospital expenditure (USD, 27,427 ± 21,316 vs. 15,282 ± 5,957; p = 0.018). Jacoby et al. [81] published their experience in 21 patients (mostly type III) with a 90% R0 rate, a median operative time of 458 min, and an estimated blood loss of 150 mL, without any complication.
Although the complications are similar in comparison to open surgery, hepatic artery pseudoaneurysm is one peculiar complication that is more common in the minimally invasive group, due to mechanical damage to the intima by excessive traction or instrumentation. This can be prevented by intrathecal separation and the use of vessel loops [77].
Recommendation: Minimal invasive surgery is a promising field, but will require further refinements. Further randomized controlled trials are required to prove its advantages over the traditional open approach, and until then, we suggest that it should be limited to tertiary care centers that have a high-volume experience of minimally invasive liver resections and pancreatic resections (Level of recommendation: III).
R0 resection is the mainstay of treatment of HC, but because of the locally advanced nature of the disease, R0 resections cannot usually be achieved. LT is a good option for such patients who havelocally advanced but nonmetastatic disease that is not amenable to resection [82]. The discouraging initial experience with tumor recurrence of 53%−84% did not help the cause, and LT was considered inappropriate for HCs [83]. A combined CRT protocol was developed by the Mayo Clinic in 1993. They tried this protocol in 19 patients, of whom 11 underwent surgery, with only 1 recurrence. They published their long-term results in 2005 on 38 patients with an 82% 5-year survival. However, this study faced strong criticism, owing to the strict selection policy of early-stage node negative and primary sclerosing cholangitis patients. Another point of criticism was 16 patients did not have tumors at operation [84,85]. The promising outcomes from the Mayo Clinic experience prompted many others to pursue LT, albeit with strict adherence to the protocol. The criteria for LT in HCs are biopsy positive or highly suspicious of malignancy, a mass lesion on imaging or CA19-9 less than 100 U/mL (at least one of these in the presence of a malignant-appearing stricture). The contraindications are tumor size more than 3 cm, nodal or metastatic disease, attempted resection or transperitoneal biopsy, and a history of malignancy within 5 years [86,87]. A multicenter study of 304 patients showed a better 5-year survival of 64% in the LT group than resection alone (18%). On subgroup analysis, the authors concluded that for node negative tumors with a size less than 3 cm, resection only is inappropriate [88].The meta-analysis by Cambridge et al. [89] in 2021 included 428 patients from 20 studies, and showed a pooled 1-, 3-, and 5-year overall survival of 77%, 55%, and 45%, respectively. The use of neoadjuvant therapy improved the overall survival at 1, 3, and 5 years to 83%, 66%, and 6%, respectively. The recurrence rate was 24 % after 3 years when neoadjuvant therapy was used (52% when no neoadjuvant therapy was used). Croome et al. [90] showed if R0 resection could be achieved, the survival was similar, regardless of treatment strategy. The benefits of neoadjuvant chemotherapy should not be forgotten, as shown by Rea et al. [85] who had examined the explanted liver, and found that 42.1% of livers did not have evidence of tumor. Similarly, Ethun et al. [88] showed a low rate of perineural invasion (33% vs. 78%) and lymph node positivity (19% vs. 38%) post-CRT, in comparison to resection alone. The impressive results obtained by neoadjuvant therapy and subsequent LT have led to the introduction of a MELD exception for HC in the U.S, (UNOS), and also in Eurotransplant [91,92]. However, the drop-out rate post-neoadjuvant therapy, due to progression of disease, ranges (0%−66%) [93]. An ongoing multicentric randomized trial in France is comparing capecitabine-based CRT with subsequent LT to standard liver and extrahepatic bile duct resection (NCT022322932; TRANSPHIL).
Recommendation: LT should be an option for unresectable non-metastatic HCs, and resection should be done if a negative margin can be achieved because of organ scarcity (Level of recommendation: II).
HC is an aggressive tumor, and an R0 resection remains the main treatment modality. Major hepatic resection is almost always required to achieve R0 with a risk of post-hepatectomy liver failure, which can be mitigated by improving the FLR by PVE and preoperative biliary drainage of the FLR. Vascular invasion is not a contraindication for resection. Portal vein resection is routinely performed in experienced centers; however, arterial resection and reconstruction is associated with increased morbidity, and should rarely be undertaken. Minimally-invasive surgery is still in the evolving stage. LT showed promising outcomes in a carefully selected subset of patients. Table 1 shows the morbidity, mortality, and 5-year overall survival of each surgical procedure [62,94-98].
REFERENCES
1. de Jong MC, Marques H, Clary BM, Bauer TW, Marsh JW, Ribero D, et al. 2012; The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer. 118:4737–4747. DOI: 10.1002/cncr.27492. PMID: 22415526.
2. Altemeier WA, Gall EA, Zinninger MM, Hoxworth PI. 1957; Sclerosing carcinoma of the major intrahepatic bile ducts. AMA Arch Surg. 75:450–460. discussion 460–461. DOI: 10.1001/archsurg.1957.01280150140015. PMID: 13457619.
3. Klatskin G. 1965; Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med. 38:241–256. DOI: 10.1016/0002-9343(65)90178-6. PMID: 14256720.
4. Saxena A, Chua TC, Chu FC, Morris DL. 2011; Improved outcomes after aggressive surgical resection of hilar cholangiocarcinoma: a critical analysis of recurrence and survival. Am J Surg. 202:310–320. DOI: 10.1016/j.amjsurg.2010.08.041. PMID: 21871986.
5. Choi JY, Kim MJ, Lee JM, Kim KW, Lee JY, Han JK, et al. 2008; Hilar cholangiocarcinoma: role of preoperative imaging with sonography, MDCT, MRI, and direct cholangiography. AJR Am J Roentgenol. 191:1448–1457. DOI: 10.2214/AJR.07.3992. PMID: 18941084.
6. Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BSJ, et al. 2001; Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 234:507–517. discussion 517–519. DOI: 10.1097/00000658-200110000-00010. PMID: 11573044. PMCID: PMC1422074.
7. Xiang S, Lau WY, Chen XP. 2015; Hilar cholangiocarcinoma: controversies on the extent of surgical resection aiming at cure. Int J Colorectal Dis. 30:159–171. DOI: 10.1007/s00384-014-2063-z. PMID: 25376337. PMCID: PMC4304009.
8. Bismuth H, Nakache R, Diamond T. 1992; Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 215:31–38. DOI: 10.1097/00000658-199201000-00005. PMID: 1309988. PMCID: PMC1242367.
9. Blechacz BR, Sanchez W, Gores GJ. 2009; A conceptual proposal for staging ductal cholangiocarcinoma. Curr Opin Gastroenterol. 25:238–239. DOI: 10.1097/MOG.0b013e3283292383. PMID: 19387257. PMCID: PMC2898128.
10. Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, et al. 2011; New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 53:1363–1371. DOI: 10.1002/hep.24227. PMID: 21480336.
11. Gaspersz MP, Buettner S, van Vugt JLA, de Jonge J, Polak WG, Doukas M, et al. 2020; Evaluation of the New American Joint Committee on Cancer staging manual 8th edition for perihilar cholangiocarcinoma. J Gastrointest Surg. 24:1612–1618. DOI: 10.1007/s11605-019-04127-x. PMID: 30756314. PMCID: PMC7359130.
12. Fausto N, Campbell JS, Riehle KJ. 2006; Liver regeneration. Hepatology. 43(2 Suppl 1):S45–S53. DOI: 10.1002/hep.20969. PMID: 16447274. PMCID: PMC10368377.
13. Schweizer W, Duda P, Tanner S, Balsiger D, Höflin F, Blumgart LH, et al. 1995; Experimental atrophy/hypertrophy complex (AHC) of the liver: portal vein, but not bile duct obstruction, is the main driving force for the development of AHC in the rat. J Hepatol. 23:71–78. DOI: 10.1016/0168-8278(95)80313-0. PMID: 8530812.
14. May BJ, Madoff DC. 2012; Portal vein embolization: rationale, technique, and current application. Semin Intervent Radiol. 29:81–89. DOI: 10.1055/s-0032-1312568. PMID: 23729977. PMCID: PMC3444878.
15. Maundura M, Koea JB. Abdeldayem HM, editor. 2017. Assessment and optimization of the future liver remnant. Updates in liver cancer. IntechOpen;p. 165. DOI: 10.5772/66139.
16. Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, et al. 1990; Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 107:521–527. PMID: 2333592.
17. Ikeda K, Kinoshita H, Hirohashi K, Kubo S, Kaneda K. 1995; The ultrastructure, kinetics and intralobular distribution of apoptotic hepatocytes after portal branch ligation with special reference to their relationship to necrotic hepatocytes. Arch Histol Cytol. 58:171–184. DOI: 10.1679/aohc.58.171. PMID: 7576869.
18. Di Stefano DR, de Baere T, Denys A, Hakime A, Gorin G, Gillet M, et al. 2005; Preoperative percutaneous portal vein embolization: evaluation of adverse events in 188 patients. Radiology. 234:625–630. DOI: 10.1148/radiol.2342031996. PMID: 15591428.
19. Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. 2001; Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 34:267–272. DOI: 10.1053/jhep.2001.26513. PMID: 11481611.
20. van Gulik TM, van den Esschert JW, de Graaf W, van Lienden KP, Busch OR, Heger M, et al. 2008; Controversies in the use of portal vein embolization. Dig Surg. 25:436–444. DOI: 10.1159/000184735. PMID: 19212116.
21. van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, et al. 2013; Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 36:25–34. DOI: 10.1007/s00270-012-0440-y. PMID: 22806245. PMCID: PMC3549243.
22. Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo F, Ueno S, et al. 2007; Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 48:721–727. DOI: 10.1080/02841850701424514. PMID: 17729001.
23. Nagino M, Ando M, Kamiya J, Uesaka K, Sano T, Nimura Y. 2001; Liver regeneration after major hepatectomy for biliary cancer. Br J Surg. 88:1084–1091. DOI: 10.1046/j.0007-1323.2001.01832.x. PMID: 11488794.
24. Kishi Y, Madoff DC, Abdalla EK, Palavecino M, Ribero D, Chun YS, et al. 2008; Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery. 144:744–751. DOI: 10.1016/j.surg.2008.05.015. PMID: 19081016. PMCID: PMC5901738.
25. van den Esschert JW, de Graaf W, van Lienden KP, Busch OR, Heger M, van Delden OM, et al. 2009; Volumetric and functional recovery of the remnant liver after major liver resection with prior portal vein embolization: recovery after PVE and liver resection. J Gastrointest Surg. 13:1464–1469. DOI: 10.1007/s11605-009-0929-0. PMID: 19475462. PMCID: PMC2710489.
26. Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, et al. ALPPS Registry Group. 2014; Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 260:829–836. discussion 836–838. DOI: 10.1097/SLA.0000000000000947. PMID: 25379854.
27. Olthof PB, Coelen RJS, Wiggers JK, Groot Koerkamp B, Malago M, Hernandez-Alejandro R, et al. 2017; High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford). 19:381–387. DOI: 10.1016/j.hpb.2016.10.008. PMID: 28279621. PMCID: PMC5662942.
28. Hernandez-Alejandro R, Bertens KA, Pineda-Solis K, Croome KP. 2015; Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery. 157:194–201. DOI: 10.1016/j.surg.2014.08.041. PMID: 25282528.
29. Petrowsky H, Györi G, de Oliveira M, Lesurtel M, Clavien PA. 2015; Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg. 261:e90–e92. DOI: 10.1097/SLA.0000000000001087. PMID: 25706390.
30. Alvarez FA, Ardiles V, Sanchez Claria R, Pekolj J, de Santibañes E. 2013; Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg. 17:814–821. DOI: 10.1007/s11605-012-2092-2. PMID: 23188224.
31. Gauzolino R, Castagnet M, Blanleuil ML, Richer JP. 2013; The ALPPS technique for bilateral colorectal metastases: three "variations on a theme". Updates Surg. 65:141–148. DOI: 10.1007/s13304-013-0214-3. PMID: 23690242.
32. Li J, Kantas A, Ittrich H, Koops A, Achilles EG, Fischer L, et al. 2016; Avoid "all-touch" by hybrid ALPPS to achieve oncological efficacy. Ann Surg. 263:e6–e7. DOI: 10.1097/SLA.0000000000000845. PMID: 25072445.
33. Robles R, Parrilla P, López-Conesa A, Brusadin R, de la Peña J, Fuster M, et al. 2014; Tourniquet modification of the associating liver partition and portal ligation for staged hepatectomy procedure. Br J Surg. 101:1129–1134. discussion 1134DOI: 10.1002/bjs.9547. PMID: 24947768.
34. Gall TM, Sodergren MH, Frampton AE, Fan R, Spalding DR, Habib NA, et al. 2015; Radio-frequency-assisted liver partition with portal vein ligation (RALPP) for liver regeneration. Ann Surg. 261:e45–e46. DOI: 10.1097/SLA.0000000000000607. PMID: 24670841.
35. Gringeri E, Boetto R, D'Amico FE, Bassi D, Cillo U. 2015; Laparoscopic microwave ablation and portal vein ligation for staged hepatectomy (LAPS): a minimally invasive first-step approach. Ann Surg. 261:e42–e43. DOI: 10.1097/SLA.0000000000000606. PMID: 24651131.
36. Teng F, Tang YY, Dai JL, Li Y, Chen ZY. 2020; The effect and safety of preoperative biliary drainage in patients with hilar cholangiocarcinoma: an updated meta-analysis. World J Surg Oncol. 18:174. DOI: 10.1186/s12957-020-01904-w. PMID: 32682432. PMCID: PMC7368977.
37. Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. 1990; Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 14:535–543. discussion 544DOI: 10.1007/BF01658686. PMID: 2166381.
38. Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, et al. 1990; Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 107:521–527. PMID: 2333592.
39. Wiggers JK, Groot Koerkamp B, Cieslak KP, Doussot A, van Klaveren D, Allen PJ, et al. 2016; Postoperative mortality after liver resection for perihilar cholangiocarcinoma: development of a risk score and importance of biliary drainage of the future liver remnant. J Am Coll Surg. 223:321–331.e1. DOI: 10.1016/j.jamcollsurg.2016.03.035. PMID: 27063572. PMCID: PMC4961586.
40. Kennedy TJ, Yopp A, Qin Y, Zhao B, Guo P, Liu F, et al. 2009; Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB (Oxford). 11:445–451. DOI: 10.1111/j.1477-2574.2009.00090.x. PMID: 19768150. PMCID: PMC2742615.
41. Wronka KM, Grąt M, Stypułkowski J, Bik E, Patkowski W, Krawczyk M, et al. 2019; Relevance of preoperative hyperbilirubinemia in patients undergoing hepatobiliary resection for hilar cholangiocarcinoma. J Clin Med. 8:458. DOI: 10.3390/jcm8040458. PMID: 30959757. PMCID: PMC6517893.
42. Paik WH, Loganathan N, Hwang JH. 2014; Preoperative biliary drainage in hilar cholangiocarcinoma: when and how? World J Gastrointest Endosc. 6:68–73. DOI: 10.4253/wjge.v6.i3.68. PMID: 24634710. PMCID: PMC3952162.
43. Hartog H, Ijzermans JN, van Gulik TM, Groot Koerkamp B. 2016; Resection of perihilar cholangiocarcinoma. Surg Clin North Am. 96:247–267. DOI: 10.1016/j.suc.2015.12.008. PMID: 27017863.
44. Bird N, Elmasry M, Jones R, Elniel M, Kelly M, Palmer D, et al. 2017; Role of staging laparoscopy in the stratification of patients with perihilar cholangiocarcinoma. Br J Surg. 104:418–425. DOI: 10.1002/bjs.10399. PMID: 27861766.
45. Coelen RJ, Ruys AT, Wiggers JK, Nio CY, Verheij J, Gouma DJ, et al. 2016; Development of a risk score to predict detection of metastasized or locally advanced perihilar cholangiocarcinoma at staging laparoscopy. Ann Surg Oncol. 23(Suppl 5):904–910. DOI: 10.1245/s10434-016-5531-6. PMID: 27586005. PMCID: PMC5149561.
46. Mantel HT, Westerkamp AC, Sieders E, Peeters PM, de Jong KP, Boer MT, et al. 2016; Intraoperative frozen section analysis of the proximal bile ducts in hilar cholangiocarcinoma is of limited value. Cancer Med. 5:1373–1380. DOI: 10.1002/cam4.693. PMID: 27062713. PMCID: PMC4944862.
47. Ribero D, Amisano M, Lo Tesoriere R, Rosso S, Ferrero A, Capussotti L. 2011; Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma. Ann Surg. 254:776–781. discussion 781–83. DOI: 10.1097/SLA.0b013e3182368f85. PMID: 22042470.
48. Ke Q, Chen Y, Huang Q, Lin N, Wang L, Liu J. 2020; Does additional resection of a positive microscopic ductal margin benefit patients with perihilar cholangiocarcinoma: a systematic review and meta-analysis. PLoS One. 15:e0232590. DOI: 10.1371/journal.pone.0232590. PMID: 32379819. PMCID: PMC7205232.
49. Nooijen LE, Franken LC, de Boer MT, Buttner S, van Dieren S, Koerkamp BG, et al. 2022; Value of routine intraoperative frozen sections of proximal bile duct margins in perihilar cholangiocarcinoma, a retrospective multicenter and matched case-control study. Eur J Surg Oncol. 48:2424–2431. DOI: 10.1016/j.ejso.2022.06.011. PMID: 35729016.
50. DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. 2007; Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 245:755–762. DOI: 10.1097/01.sla.0000251366.62632.d3. PMID: 17457168. PMCID: PMC1877058.
51. Endo I, House MG, Klimstra DS, Gönen M, D'Angelica M, Dematteo RP, et al. 2008; Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol. 15:2104–2112. DOI: 10.1245/s10434-008-0003-2. PMID: 18543039.
52. Seyama Y, Kubota K, Sano K, Noie T, Takayama T, Kosuge T, et al. 2003; Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 238:73–83. DOI: 10.1097/01.SLA.0000074960.55004.72. PMID: 12832968. PMCID: PMC1422671.
53. Sakamoto E, Nimura Y, Hayakawa N, Kamiya J, Kondo S, Nagino M, et al. 1998; The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg. 227:405–411. DOI: 10.1097/00000658-199803000-00013. PMID: 9527064. PMCID: PMC1191279.
54. Launois B, Terblanche J, Lakehal M, Catheline JM, Bardaxoglou E, Landen S, et al. 1999; Proximal bile duct cancer: high resectability rate and 5-year survival. Ann Surg. 230:266–275. DOI: 10.1097/00000658-199908000-00018. PMID: 10450742. PMCID: PMC1420870.
55. Otani K, Chijiiwa K, Kai M, Ohuchida J, Nagano M, Kondo K. 2012; Role of hilar resection in the treatment of hilar cholangiocarcinoma. Hepatogastroenterology. 59:696–700. DOI: 10.5754/hge09725. PMID: 22469711.
56. Ikeyama T, Nagino M, Oda K, Ebata T, Nishio H, Nimura Y. 2007; Surgical approach to bismuth type I and II hilar cholangiocarcinomas: audit of 54 consecutive cases. Ann Surg. 246:1052–1057. DOI: 10.1097/SLA.0b013e318142d97e. PMID: 18043110.
57. Lim JH, Choi GH, Choi SH, Kim KS, Choi JS, Lee WJ. 2013; Liver resection for Bismuth type I and type II hilar cholangiocarcinoma. World J Surg. 37:829–837. DOI: 10.1007/s00268-013-1909-9. PMID: 23354922.
58. Hosokawa I, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, et al. 2014; Surgical strategy for hilar cholangiocarcinoma of the left-side predominance: current role of left trisectionectomy. Ann Surg. 259:1178–1185. DOI: 10.1097/SLA.0000000000000584. PMID: 24509210.
59. Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, et al. 2013; Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 258:129–140. DOI: 10.1097/SLA.0b013e3182708b57. PMID: 23059502.
60. Jo HS, Kim DS, Yu YD, Kang WH, Yoon KC. 2020; Right-side versus left-side hepatectomy for the treatment of hilar cholangiocarcinoma: a comparative study. World J Surg Oncol. 18:3. DOI: 10.1186/s12957-019-1779-1. PMID: 31901228. PMCID: PMC6942359.
61. Bengmark S, Ekberg H, Evander A, Klofver-Stahl B, Tranberg KG. 1988; Major liver resection for hilar cholangiocarcinoma. Ann Surg. 207:120–125. DOI: 10.1097/00000658-198802000-00002. PMID: 2829760. PMCID: PMC1493364.
62. Yang M, Li WW, Chen JH, Cui MH, Liu JL. 2021; The value of caudate lobectomy in hilar cholangiocarcinoma treatment: a meta-analysis. Medicine (Baltimore). 100:e24727. DOI: 10.1097/MD.0000000000024727. PMID: 33607815. PMCID: PMC7899860.
63. Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y, et al. 2012; Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann Surg. 256:297–305. DOI: 10.1097/SLA.0b013e31826029ca. PMID: 22750757.
64. Neuhaus P, Thelen A, Jonas S, Puhl G, Denecke T, Veltzke-Schlieker W, et al. 2012; Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol. 19:1602–1608. DOI: 10.1245/s10434-011-2077-5. PMID: 21964888.
65. Wu XS, Dong P, Gu J, Li ML, Wu WG, Lu JH, et al. 2013; Combined portal vein resection for hilar cholangiocarcinoma: a meta-analysis of comparative studies. J Gastrointest Surg. 17:1107–1115. DOI: 10.1007/s11605-013-2202-9. PMID: 23592188.
66. Anderson B, Doyle MBM. 2019; Surgical considerations of hilar cholangiocarcinoma. Surg Oncol Clin N Am. 28:601–617. DOI: 10.1016/j.soc.2019.06.003. PMID: 31472908.
67. Nagino M, Nimura Y, Nishio H, Ebata T, Igami T, Matsushita M, et al. 2010; Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann Surg. 252:115–123. DOI: 10.1097/SLA.0b013e3181e463a7. PMID: 20531001.
68. Rela M, Rajalingam R, Shanmugam V, O' Sullivan A, Reddy MS, Heaton N. 2014; Novel en-bloc resection of locally advanced hilar cholangiocarcinoma: the Rex recess approach. Hepatobiliary Pancreat Dis Int. 13:93–97. DOI: 10.1016/S1499-3872(14)60013-8. PMID: 24463086.
69. Li J, Zhou MH, Ma WJ, Li FY, Deng YL. 2020; Extended lymphadenectomy in hilar cholangiocarcinoma: what it will bring? World J Gastroenterol. 26:3318–3325. DOI: 10.3748/wjg.v26.i24.3318. PMID: 32655260. PMCID: PMC7327786.
70. Bagante F, Tran T, Spolverato G, Ruzzenente A, Buttner S, Ethun CG, et al. 2016; Perihilar cholangiocarcinoma: number of nodes examined and optimal lymph node prognostic scheme. J Am Coll Surg. 222:750–759.e2. DOI: 10.1016/j.jamcollsurg.2016.02.012. PMID: 27113512. PMCID: PMC5450030.
71. Kitagawa Y, Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, et al. 2001; Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 233:385–392. DOI: 10.1097/00000658-200103000-00013. PMID: 11224627. PMCID: PMC1421255.
72. Aoba T, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, et al. 2013; Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann Surg. 257:718–725. DOI: 10.1097/SLA.0b013e3182822277. PMID: 23407295.
73. Mantel HT, Wiggers JK, Verheij J, Doff JJ, Sieders E, van Gulik TM, et al. 2015; Lymph node micrometastases are associated with worse survival in patients with otherwise node-negative hilar cholangiocarcinoma. Ann Surg Oncol. 22 Suppl 3:S1107–S1115. DOI: 10.1245/s10434-015-4723-9. PMID: 26178761. PMCID: PMC4686550.
74. Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, et al. 2018; Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg. 267:199–207. DOI: 10.1097/SLA.0000000000002353. PMID: 28657937.
75. Yu H, Wu SD, Chen DX, Zhu G. 2011; Laparoscopic resection of Bismuth type I and II hilar cholangiocarcinoma: an audit of 14 cases from two institutions. Dig Surg. 28:44–49. DOI: 10.1159/000322398. PMID: 21293131.
76. Gumbs AA, Jarufe N, Gayet B. 2013; Minimally invasive approaches to extrapancreatic cholangiocarcinoma. Surg Endosc. 27:406–414. DOI: 10.1007/s00464-012-2489-8. PMID: 22926892.
77. Zhang Y, Dou C, Wu W, Liu J, Jin L, Hu Z, et al. 2020; Total laparoscopic versus open radical resection for hilar cholangiocarcinoma. Surg Endosc. 34:4382–4387. DOI: 10.1007/s00464-019-07211-0. PMID: 31664578.
78. Ratti F, Fiorentini G, Cipriani F, Catena M, Paganelli M, Aldrighetti L. 2020; Perihilar cholangiocarcinoma: are we ready to step towards minimally invasiveness? Updates Surg. 72:423–433. DOI: 10.1007/s13304-020-00752-3. PMID: 32221907.
79. He YG, Huang W, Ren Q, Li J, Yang FX, Deng CL, et al. 2022; Comparison of efficacy and safety between laparoscopic and open radical resection for hilar cholangiocarcinoma-a propensity score-matching analysis. Front Oncol. 12:1004974. DOI: 10.3389/fonc.2022.1004974. PMID: 36226051. PMCID: PMC9549331.
80. Xu Y, Wang H, Ji W, Tang M, Li H, Leng J, et al. 2016; Robotic radical resection for hilar cholangiocarcinoma: perioperative and long-term outcomes of an initial series. Surg Endosc. 30:3060–3070. DOI: 10.1007/s00464-016-4925-7. PMID: 27194255.
81. Jacoby H, Rayman S, Ross S, Crespo K, Syblis C, Rosemurgy A, et al. 2022; Robotic resection of hilar cholangiocarcinoma: a single institution experience. Mini-invasive Surg. 6:58. DOI: 10.20517/2574-1225.2022.58.
82. Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. 2015; Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 17:691–699. DOI: 10.1111/hpb.12450. PMID: 26172136. PMCID: PMC4527854.
83. Iwatsuki S, Todo S, Marsh JW, Madariaga JR, Lee RG, Dvorchik I, et al. 1998; Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 187:358–364. DOI: 10.1016/S1072-7515(98)00207-5. PMID: 9783781. PMCID: PMC2991118.
84. De Vreede I, Steers JL, Burch PA, Rosen CB, Gunderson LL, Haddock MG, et al. 2000; Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 6:309–316. DOI: 10.1053/lv.2000.6143. PMID: 10827231.
85. Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, et al. 2005; Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 242:451–458. discussion 458–461. DOI: 10.1097/01.sla.0000179678.13285.fa. PMID: 16135931. PMCID: PMC1357753.
86. Gores GJ, Gish RG, Sudan D, Rosen CB. MELD Exception Study Group. 2006; Model for end-stage liver disease (MELD) exception for cholangiocarcinoma or biliary dysplasia. Liver Transpl. 12(12 Suppl 3):S95–S97. DOI: 10.1002/lt.20965. PMID: 17123289.
87. Zaydfudim VM, Rosen CB, Nagorney DM. 2014; Hilar cholangiocarcinoma. Surg Oncol Clin N Am. 23:247–263. DOI: 10.1016/j.soc.2013.10.005. PMID: 24560109.
88. Ethun CG, Lopez-Aguiar AG, Anderson DJ, Adams AB, Fields RC, Doyle MB, et al. 2018; Transplantation versus resection for hilar cholangiocarcinoma: an argument for shifting treatment paradigms for resectable disease. Ann Surg. 267:797–805. DOI: 10.1097/SLA.0000000000002574. PMID: 29064885. PMCID: PMC6002861.
89. Cambridge WA, Fairfield C, Powell JJ, Harrison EM, Søreide K, Wigmore SJ, et al. 2021; Meta-analysis and meta-regression of survival after liver transplantation for unresectable perihilar cholangiocarcinoma. Ann Surg. 273:240–250. DOI: 10.1097/SLA.0000000000003801. PMID: 32097164.
90. Croome KP, Rosen CB, Heimbach JK, Nagorney DM. 2015; Is liver transplantation appropriate for patients with potentially resectable de novo hilar cholangiocarcinoma? J Am Coll Surg. 221:130–139. DOI: 10.1016/j.jamcollsurg.2015.01.064. PMID: 25872685.
91. Gores GJ, Gish RG, Sudan D, Rosen CB. MELD Exception Study Group. 2006; Model for end-stage liver disease (MELD) exception for cholangiocarcinoma or biliary dysplasia. Liver Transpl. 12(12 Suppl 3):S95–S97. DOI: 10.1002/lt.20965. PMID: 17123289.
92. Umgelter A, Hapfelmeier A, Kopp W, van Rosmalen M, Rogiers X, Guba M. Eurotransplant Liver Advisory Committee. 2017; Disparities in Eurotransplant liver transplantation wait-list outcome between patients with and without model for end-stage liver disease exceptions. Liver Transpl. 23:1256–1265. DOI: 10.1002/lt.24805. PMID: 28650098.
93. Lang SA, Bednarsch J, Czigany Z, Joechle K, Kroh A, Amygdalos I, et al. 2021; Liver transplantation in malignant disease. World J Clin Oncol. 12:623–645. DOI: 10.5306/wjco.v12.i8.623. PMID: 34513597. PMCID: PMC8394155.
94. Soares KC, Jarnagin WR. 2021; The landmark series: hilar cholangiocarcinoma. Ann Surg Oncol. 28:4158–4170. DOI: 10.1245/s10434-021-09871-6. PMID: 33829358. PMCID: PMC9273057.
95. Kim KB, Choi DW, Heo JS, Han IW, Shin SH, You Y, et al. 2021; The impact of portal vein resection on outcome of hilar cholangiocarcinoma. Ann Hepatobiliary Pancreat Surg. 25:221–229. DOI: 10.14701/ahbps.2021.25.2.221. PMID: 34053925. PMCID: PMC8180392.
96. Rebelo A, Friedrichs J, Grilli M, Wahbeh N, Partsakhashvili J, Ukkat J, et al. 2022; Systematic review and meta-analysis of surgery for hilar cholangiocarcinoma with arterial resection. HPB (Oxford). 24:1600–1614. DOI: 10.1016/j.hpb.2022.04.002. PMID: 35490097.
97. Fancellu A, Sanna V, Deiana G, Ninniri C, Turilli D, Perra T, et al. 2021; Current role of hepatopancreatoduodenectomy for the management of gallbladder cancer and extrahepatic cholangiocarcinoma: a systematic review. World J Gastrointest Oncol. 13:625–637. DOI: 10.4251/wjgo.v13.i6.625. PMID: 34163578. PMCID: PMC8204357.
98. Dopazo C, Charco R. 2023; Liver transplantation for perihilar cholangiocarcinoma. Do we need to move forward? Hepatoma Res. 9:10. DOI: 10.20517/2394-5079.2022.75.
Fig. 1
(A) MRI showing duct separation, suggesting a hilar block. (B) Triphasic CT showing hilar cholangiocarcinoma near the portal vein.
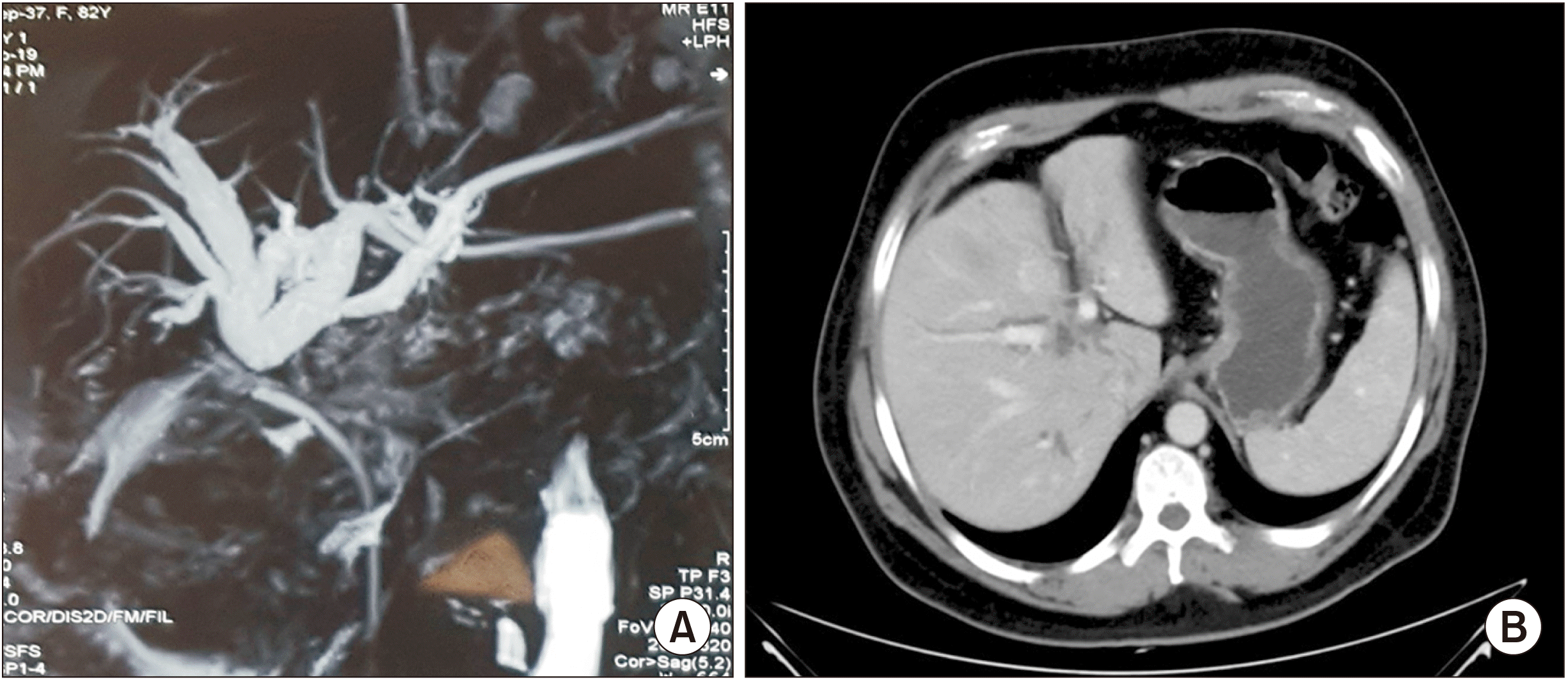
Fig. 2
(A, B) Intra-operative picture showing hypertrophied left lobe with atrophied right lobe 2 weeks following portal vein embolization. This patient underwent right-trisectionectomy for type IIIa hilar cholangiocarcinoma (HC). (C) Post-right-trisectionectomy specimen showing HC grasped by the metal forceps.
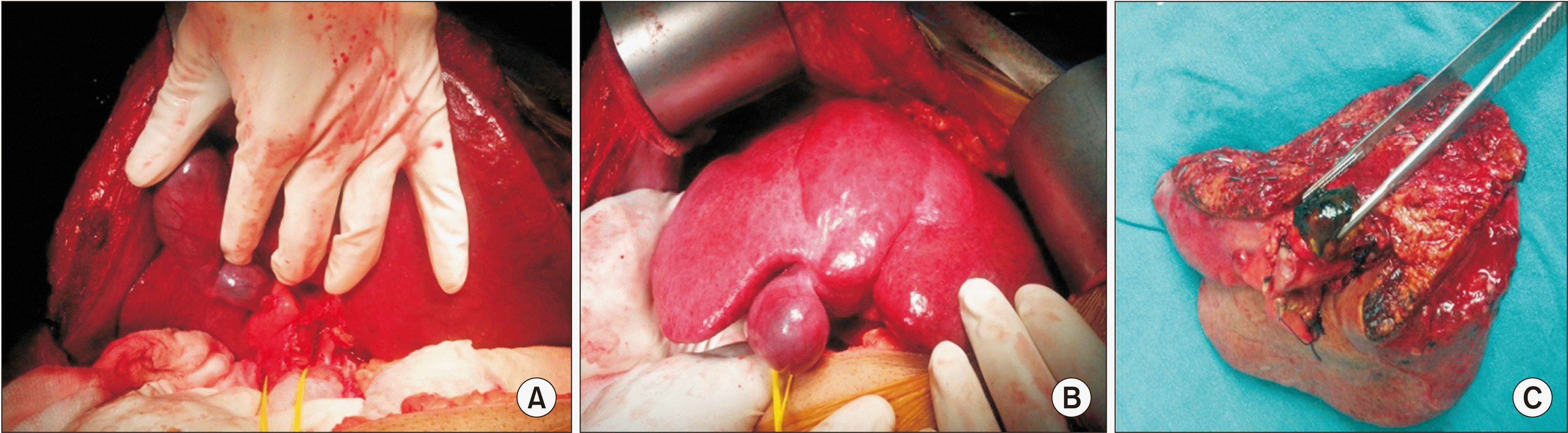
Fig. 3
(A) Intra-operative picture showing removal of a self-expandable metallic stent (held with the metal instrument) in a patient with hilar cholangiocarcinoma who underwent left hepatectomy. (B) Removed self-expandable metallic stent.
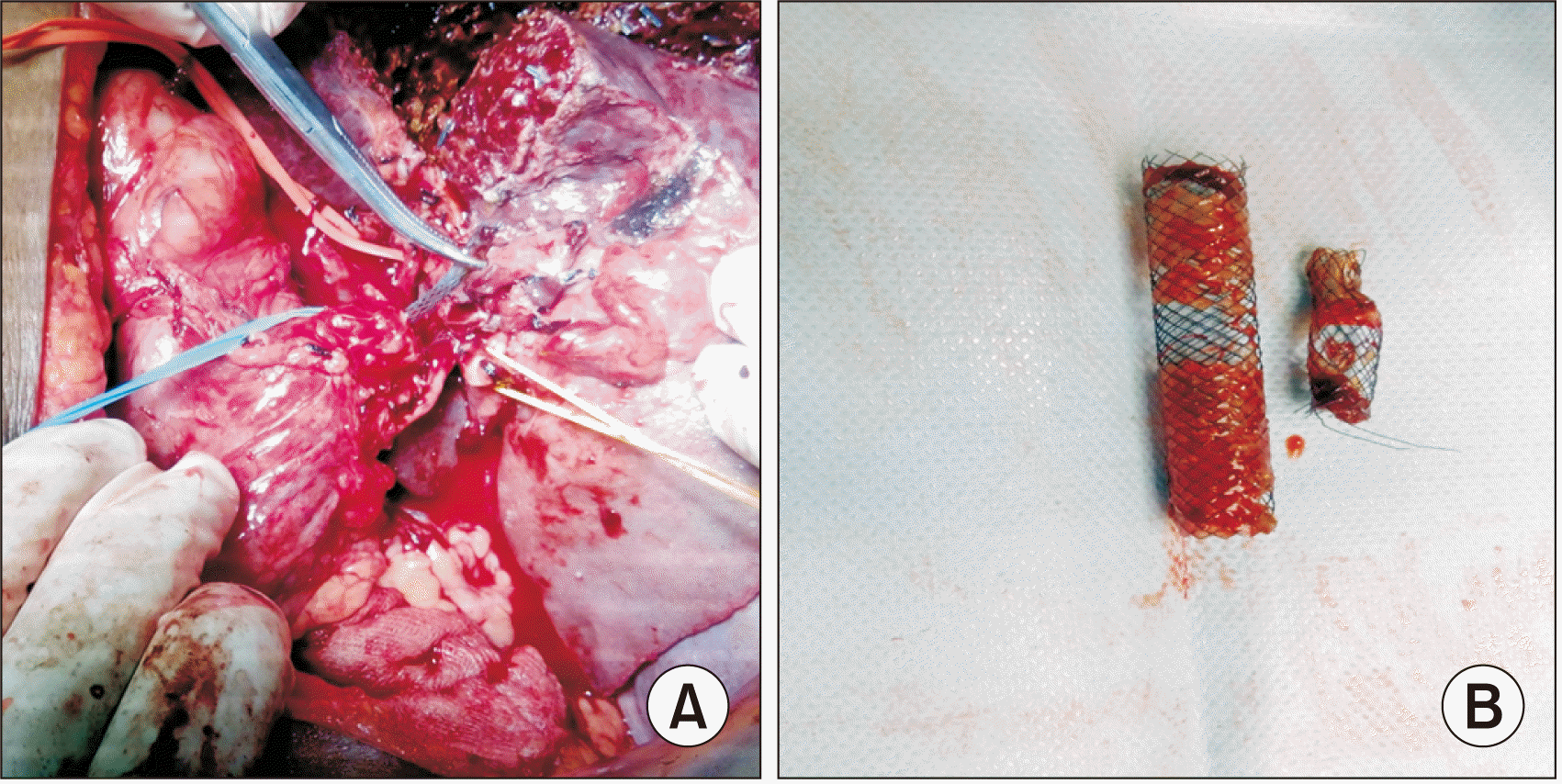
Fig. 4
(A) Staging laparoscopy showing peritoneal deposits. (B) Same patient showing ascites. Further surgery was abandoned in this patient, thus avoiding a futile laparotomy.
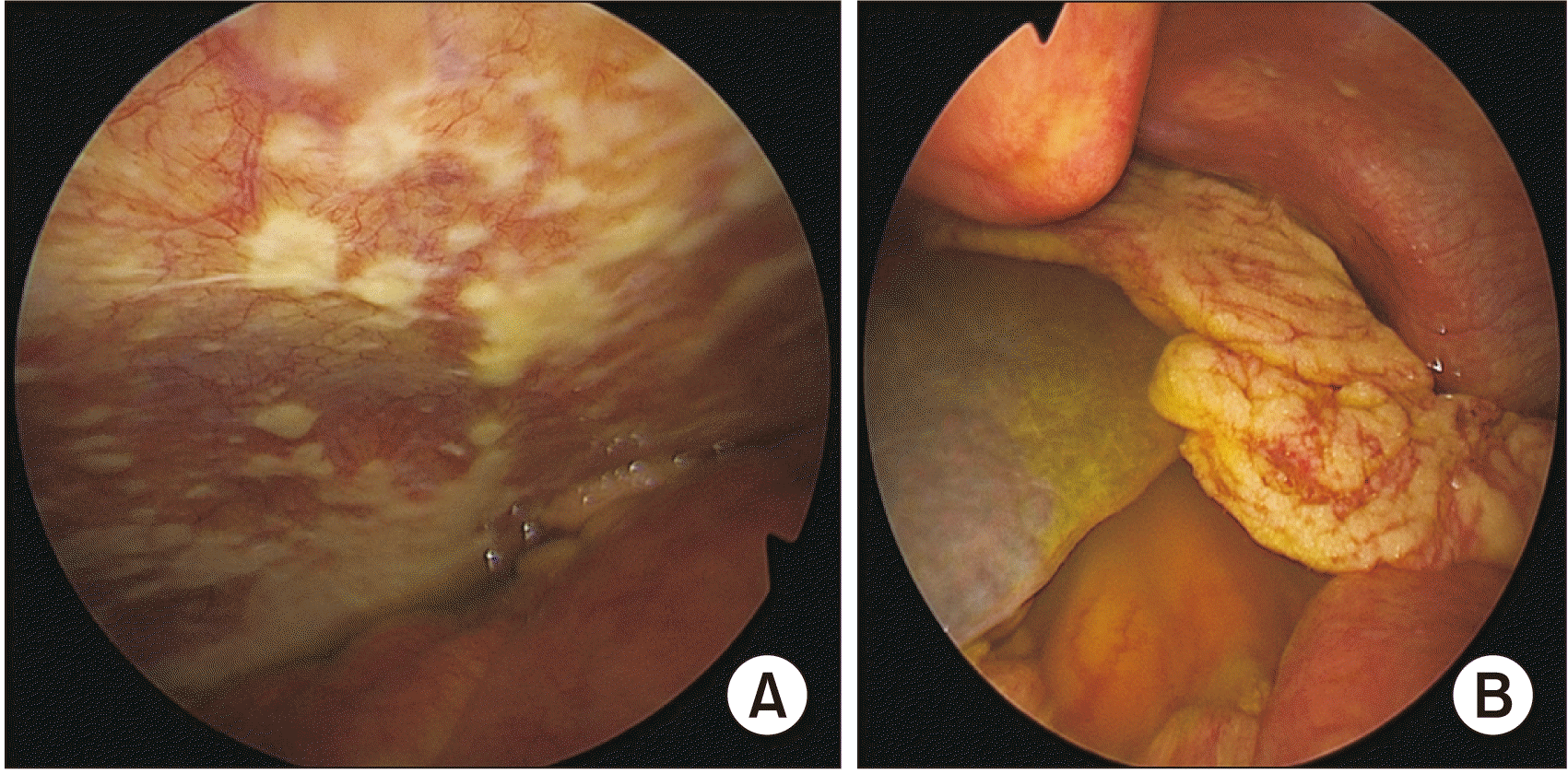
Table 1
Showing the morbidity, mortality, and 5-year overall survival of each procedure
| Procedure | Morbidity (%) | Mortality (%) | 5- year overall survival (%) |
|---|---|---|---|
| Major hepatectomy [94] | 43–65 | 2–17 | 25–40 |
| Portal vein resection [95] | 34.3 | 2.9 | 37.7 |
| Arterial and portal vein resection [96] | 52 | 6.8 | 18 |
| Caudate lobectomy [62] | 36.3 | 4.51 | 10.6–33 |
| Hepatopancreatoduodenectomy [97] | 37–97.4 | 0–34.2 | 17.9–49.2 |
| Liver transplantation [98] | 20–50 | 4.8–25 | 36–75 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download