Abstract
Objective
To compare the efficacy of home-based cardiac rehabilitation (HBCR) and center-based cardiac rehabilitation (CBCR) in cardiovascular risk factor management.
Methods
We performed retrospective review of the electronic medical records of 72 patients who were hospitalized for acute coronary syndrome and participated in a cardiac rehabilitation (CR) program for the first time. The participants were stratified into the HBCR group, receiving educational programs and performing self-exercise at home, and the CBCR group, participating in electrocardiogram monitoring monitoring exercise training in hospital settings. The results of the Lifestyle Questionnaire survey were investigated at baseline, 3 months, and 6 months.
Results
Both groups showed significant improvements in serum low-density lipoprotein levels, frequency of alcohol consumption, eating habits and psychological status. Moderate-intensity exercise duration and the maximal metabolic equivalents values improved significantly in both groups but slightly more in the CBCR group. However, the number of current smokers increased in both groups, and no significant changes were found in body mass index, serum glycated hemoglobin levels, serum high-density lipoprotein levels, or high-intensity exercise duration.
After acute coronary syndrome (ACS), all survivors need to rigorously manage their documented cardiovascular (CV) risk factors to improve long-term outcomes. Cardiac rehabilitation (CR) is the most important evidence-based intervention for the secondary prevention after ACS [1,2].
CR is categorized into three main phases as follows: Phase 1 (early mobilization during acute in-patient hospitalization), Phase 2 (rehabilitation services traditionally delivered in an outpatient setting that focus on health behavior change, risk factor modification, and psychosocial well-being), and Phase 3 (long-term maintenance of lifestyle changes) [3]. In Phase 2, patients typically receive center-based CR (CBCR) for approximately 3 months of outpatient-monitored exercise programs, but the participation rates are very low [4,5]. Therefore, home-based CR (HBCR) was introduced to expand the access and participation of patients compared with conventional CBCR [6].
According to a Cochrane Review [7], HBCR and CBCR showed similar effects in improving clinical health-related quality of life outcomes. Additionally, managing CV risk factors for patients with coronary artery disease (CAD) is as important as improving cardiorespiratory fitness (CRF) levels [8]. However many comparative studies of HBCR and CBCR have been focused on the efficacy of exercise-based CR program for increasing CRF level. Thus, it is necessary to assess the degree of CV risk factor management rather than exercise effect using CR.
This study aimed to retrospectively compare the effects of HBCR and CBCR, focusing on CV risk factor management by reviewing patients’ electronic medical records (EMRs).
We retrospectively analyzed patients’ EMRs at a single center (Inje University Sanggye Paik Hospital). Patient privacy and data confidentiality were maintained throughout the study period. The study was approved by the Institutional Review Board of Inje University Sanggye Paik Hospital (IRB No. 2022-12-001). The requirement for informed consent was waived due to the retrospective nature of the study.
Patients who met the following criteria were included in the study: (1) patients who participated in the CR program for the first time between March 1, 2020 and December 31, 2021; (2) patients who were diagnosed with ACS and underwent percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG) surgery or treated with drugs, who were diagnosed with valvular heart diseases or aortic dissection and underwent surgery, who were diagnosed with cardiomyopathy or acute heart failure and treated with drugs; and (3) those who visited the CR clinic for regular follow-up for 3 and 6 months and performed the cardiopulmonary exercise (CPX) test.
The exclusion criteria were as follows: (1) inability to ambulate due to physical problems including paralysis due to stroke, spinal cord injury, amputation, severe musculoskeletal pain, and dyspnea, among others and (2) incomplete EMRs.
Group assignments were performed at outpatient visits. The decision of whether patients would receive CBCR or HBCR program was based on the risk of exercise-related CV events set by the American Association of Cardiovascular and Pulmonary Rehabilitation [9] and the socioeconomic factors of each patient including time conflict to attend CBCR, distance between home and center, economical status, etc. High-risk patients were preferentially assigned to CBCR group and other patients were assigned to CBCR or HBCR according to each patient’s choice by their socioeconomic status. Although patients were classified as “high-risk” according to the risk classification, they were assigned to the HBCR group if they could not participate in the CR program in a hospital setting. Also, if low-risk patients who might be assigned to the HBCR group wanted a CBCR program, they were assigned to the CBCR group to exercise. Fig. 1 presents the patients’ selection flow chart.
The CR program was performed at a single institution according to the exercise prescription by exercise test using modified Bruce protocol [10]. Patients were asked to visit the CR clinic within 2 weeks after discharge for those who received the PCI and within 4 weeks after discharge for those who had surgery. At the first visit, patients were asked to undergo a series of tests, including the CPX test and a questionnaire to understand their lifestyle. Real-time recording 12-channel electrocardiography (CASE; GE-Marquette), respiratory gas analyzer (Quark CPET; COSMED Co.), automatic blood pressure and pulse monitor (TANGO M2; SunTech Medical Inc.), and treadmill (T-2100; GE-Marquette) were used in the CPX test. Several variables were measured in the CPX test, and changes of maximal metabolic equivalents (METsmax) were used to compare changes in CRF levels. If the respiratory exchange rate was not sufficiently obtained due to the patient’s poor condition or other reasons, the test was performed again on a different date so that reliable results could be obtained.
The survey used the “Lifestyle Questionnaire” adapted from the “Health Insurance Corporation Health Checkup Questionnaire” [11]. The questionnaire included past history, family history, smoking and drinking habits, and questions about recent exercise habits, eating habits, and psychological status. Smoking habits were investigated as to whether or not to quit and maintain smoking. Drinking habits were examined to determine the number of times a person drank alcohol per month. Regarding exercise habits, the number of days and minutes per week of moderate-intensity and high-intensity exercises were assessed separately. In contrast, regarding eating habits, the intake of high-cholesterol food was identified by classifying it into three stages. Body mass index (BMI) was calculated by measuring the height and weight, and blood tests, including serum glycated hemoglobin (HbA1c), low-density lipoprotein (LDL), and high-density lipoprotein (HDL), were compared by referring to the blood test results performed as follow-up tests at the outpatient clinic of the cardiology department.
The CR exercise program was structured as follows: They were asked to exercise for approximately 1 hour a day, 3 to 4 times per week. It comprised 5 minutes of warm-up stretching, 3 to 5 minutes of light cycling or walking, up to 40 minutes of exercise, and 5 to 10 minutes of cool down. Exercise programs prescribed to each patient included fast walking, treadmill exercise, power walking, cycling, and jogging, depending on exercise ability and condition. The initial exercise intensity was gradually increased step by step according to the target heart rate. The target heart rate was set to 60%–85% of the heart rate reserve value calculated using the maximum and minimal heart rates obtained from the CPX test. At every exercise session, patients were supervised and followed the direction of CR staff for keeping above 85% of target heart rate goal. The CBCR group visited the hospital for exercise under supervision and participated in 36 sessions for 3 to 4 months. The CBCR group participated in at least 10 of the 36 sessions of the CR program in the hospital over 3 to 4 months. Subsequently, the patients were encouraged to exercise at home after completion of 36 sessions in hospital setting. In the HBCR group, they received education on CR exercise methods and conducted self-exercises near their residences after their first visit to the CR clinic. Patients in the HBCR group were instructed to monitor their heart rate by wearing a smart watch or smart band or checking their radial pulse. The HBCR group exercised alone based on the training content, and self-management including exercise was completely self-sufficient.
All patients in the CR programs were asked to manage their risk factors such as smoking and diet. On their first visit, they were asked to participate in a 30-minute educational program on dietary methods. This education was conducted by a nutritionist who is in charge of dietary education for outpatients at hospital. If patients had difficulty quitting smoking, they were instructed to receive counseling from the smoking cessation center, if necessary. The patients were then asked to revisit the CR clinic at 3 and 6 months. During the revisit, patients underwent follow-up CPX tests and questionnaires. They were also encouraged to continue risk factor management by receiving feedback on how effectively they did exercise and managed risk factors compared with their first visit. The study outcome was investigated based on the CPX test results, blood test results, and lifestyle surveys between the first visit and, 3 and 6 months. In particular, we focused on comparing how well each patient’s risk factors are managed.
Data were analyzed using IBM SPSS version 25 (IBM SPSS). An independent t-test was used to compare the baseline characteristics of the two groups, including age and left ventricular ejection fraction (LVEF). Pearson’s chi-square test was used to analyze the baseline characteristics of the two groups for sex, smoking history, cardiac diagnosis, comorbidity, family history of cardiac disease, type of intervention and change in number of smokers. To determine the association between time and the parameters of both groups, a two-way repeated-measures ANOVA model was performed. The comparison of the degree of changes in METsmax values between the two groups was analyzed using an independent t-test. Statistical significance was defined as p<0.05.
Among the 499 patients who first visited the outpatient clinic during the period, 72 patients completed follow-up visits twice for 6 months. Among them, there were 40 in the CBCR group and 32 in the HBCR group. Of the 40 patients in the CBCR group, 27 (67.5%) completed 36 CBCR sessions, and those who did not complete all 36 sessions attended 24.9 sessions in average. Table 1 shows the demographic data, and a comparison between the two groups showed no significant differences (p>0.05). However, the LVEF at baseline in the HBCR group was significantly higher than that in the CBCR group (56.0%±8.0% vs. 46.9%±12.8%; p<0.001). Fewer patients with ST-segment elevation myocardial infarction were found in the HBCR group than in the CBCR group (21.9% vs. 47.5%; p=0.022). The patients were predominantly male in both groups; however the number of females was low in the HBCR group (31 male and 1 female) than in the CBCR group (27 male and 13 female; p<0.001; Table 1).
No significant change in BMI was found between baseline and follow-up, and no significant difference was found between the two groups (p>0.05; Table 2, Fig. 2).
Table 2 summarizes the changes in laboratory findings to compare the management of other CV risk factors. Serum HbA1c levels (HBCR, 7.3%±0.7% and CBCR, 7.5%±0.6% at baseline) slightly decreased at 3 and 6 months (HBCR, 6.6%±0.6% and CBCR, 7.3%±0.5%; HBCR, 6.5%±0.4% and CBCR, 7.0%±0.4%; respectively), which was the same in both groups, but not statistically significant (p>0.05). The change in serum HbA1c in the non-diabetic patients was 5.7%±0.1%, 5.8%±0.1%, 5.8%±0.1% in the CBCR group, and 5.8%±0.1%, 5.8%±0.1%, 5.9%±0.1% in the HBCR group, respectively, in chronological order. The change in serum HbA1c in the diabetic patients was 8.0%±3.5%, 7.7%±3.9%, 7.4%±2.8% in the CBCR group, and 7.6%±2.4%, 6.9%±0.4%, 6.8%±0.4% in the HBCR group, respectively, in chronological order. Changes in serum HbA1c were not statistically significant, with or without diabetes in both groups (p>0.05). Serum HDL levels at baseline (HBCR, 45.4±3.3 and CBCR, 45.0±2.5 mg/dL) did not differ between the two groups; and showed no significant changes at follow-up in both groups (HBCR, 43.9±2.8 and CBCR, 42.4±2.1 mg/dL; HBCR, 42.5±3.7 and CBCR, 45.8±2.8 mg/dL; respectively; p>0.05; Table 2, Fig. 2).
In contrast, serum LDL levels (HBCR, 102.4±11.3 and CBCR, 97.0±8.6 mg/dL at baseline) were significantly decreased at 3 months in both groups (HBCR, 62.6±6.4 and CBCR, 67.1±4.8 mg/dL, p<0.05), and the results were maintained even at 6 months (HBCR, 60.6±5.5 and CBCR, 65.8±4.2 mg/dL; p<0.05). In addition, no significant difference was found in serum LDL levels between the two groups (p>0.05; Table 2, Fig. 2).
Table 2 summarizes the changes in CPX test results and lifestyle for the HBCR and CBCR groups. When comparing the METsmax values between the two groups, the baseline values were significantly higher in the HBCR group (HBCR, 6.6±0.3 and CBCR, 5.6±0.3 mL/kg/min; p<0.05; Table 2, Fig. 2). However, both groups showed a statistically significant increase in values at 3 (HBCR, 6.9±0.3 and CBCR, 6.6±0.3 mL/kg/min; p<0.05) months and 6 (HBCR, 7.1±0.3 and CBCR, 6.5±0.3 mL/kg/min; p<0.05) months from baseline. No significant difference was found in the degree of change in the METsmax values between the two groups at 6 months (p>0.05). However at 3 months, the degree of change was significantly higher in the CBCR group (p<0.05; Table 3).
Fig. 2 shows the changes in exercise duration. High-intensity exercise duration per week did not differ between the groups at baseline (HBCR, 26.3±20.5 and CBCR, 30.5±18.4 min/week; p>0.05), and did not significantly increase at 3 (HBCR, 52.8±22.5 and CBCR, 33.6±20.9 min/week) and 6 (HBCR, 17.8±8.5 and CBCR, 16.6±7.6 min/week) months from baseline in both groups (p>0.05). Regarding the moderate-intensity exercise duration at baseline, the HBCR group (109.7±24.8 min/week) exercised more time than the CBCR group (38.1±22.2 min/week; p<0.05). However, the duration of exercise increased significantly at 3 (HBCR, 219.7±30.3 and CBCR, 132.9±27.1 min/week) and 6 (HBCR, 210.6±29.2 and CBCR, 159.9±26.1 min/week) months in both groups (p<0.05), but the change was not significantly different between the two groups (p>0.05; Table 2, Fig. 2).
Changes in lifestyle modification parameters, including frequency of alcohol consumption, eating habits, and psychological state, are shown in Fig. 2. All three parameters showed no significant differences between the two groups (p>0.05) and were dramatically improved during the follow-up period (p<0.05). The frequency of drinking and consuming high-cholesterol foods decreased, and psychological stability was achieved (Table 2, Fig. 2).
However, the number of current smokers increased in both groups at 6 months (HBCR, 5→8 and CBCR, 2→3), but more in the HBCR group (Table 2).
CR significantly reduces secondary CV events and mortality, and it is a class 1A recommendation by the American Heart Association and the American College of Cardiology [12]. However, many patients prefer HBCR over CBCR because of the lack of accessibility and time. Although HBCR can potentially expand patient access and participation, there is concern that inadequate direct supervision and lack of physical interaction with CR staff will reduce the physical and psychological benefits demonstrated by CBCR [12]. Ornish et al. [13] showed that the rate of coronary heart disease progression doubled over 5 years if intensive lifestyle changes were not made. Therefore, managing and maintaining risk factors for CR is as important as maintaining CRF levels. In the CBCR group, patients visited the center up to 36 times and exercised under supervision, whereas exercise training and risk factor management were entirely left to the patients in the HBCR group. Although feedback was provided through periodic outpatient follow-ups every 3 months, it was necessary to determine whether the risk factor management was effective in the patient undergoing HBCR, who had to manage themselves from the beginning after onset. The purpose of this study was to confirm how CV risk factors were effectively managed in the HBCR compared to CBCR groups.
Our study has several important findings. First, lifestyle habits were greatly improved and effectively maintained in both groups. Additionally, in both groups, after commencing CR, the serum LDL level significantly decreased, the intake of high-cholesterol foods and alcohol decreased, and anxiety or depressive psychological conditions stabilized at 3 months. Notably, these results were maintained for approximately 6 months.
The guidelines of the European Society of Cardiology and the European Atherosclerosis Society recommend a LDL-C target value of <70 mg/dL (1.8 mmol/L). In Bernhard’s study, participation in CR in Germany improved the control of modifiable CV risk factors, specifically LDL-C, in patients after acute myocardial infarction [14]. Sorting out the relative effects of CR and lipid therapy can be difficult, but Snow et al. suggested participation in CR significantly potentiates the lipid-improving effects of pharmacological therapy [15,16]. All patients who participated in our CR program were given a training session on diet, and the results showed that most patients ate less high-cholesterol foods. In our CR programs, serum LDL levels could be lowered by correcting eating habits combined with exercise. However, because of the nature of the CR program, which was centered on aerobic exercise, a significant change in serum HDL level could not be expected, and it was difficult to expect a significant change in serum HbA1c level in a short period.
A significant reduction in the amount of alcohol consumed was also observed in our study. Athyros et al. [17] reported that heavy drinking was associated with an increase in the prevalence of metabolic syndrome, CAD, stroke, and peripheral arterial disease. In another study of alcohol-consuming populations, the amount of alcohol consumption significantly impacted blood pressure values, hypertension prevalence, and CV and all-cause mortality [18]. In addition, patients’ psychological states were stabilized in our CR program. Among patients with CAD, acute psychological stress has been shown to induce transient myocardial ischemia, and long-term stress can increase the risk of recurrent ACS events and mortality [19]. Anxiety of patients was improved by explaining to them how much their CRF level was and how much it had improved based on the results of the CPX test. Therefore, through these lifestyle modifications, the effect of preventing the recurrence of CV disease in patients can be expected in CR program.
Second, we observed a marked improvement in patients’ exercise habits. Comparing the time spent on moderate-intensity exercise for 1 week, both groups showed a significant increase in exercise duration at 3 and 6 months. Exercise duration increased by approximately 2 times in the HBCR group and 3 times in the CBCR group. The change in exercise time was slightly smaller in the HBCR group, which may be because the exercise time at baseline was significantly greater in the HBCR group than in the CBCR group. These baseline differences are believed to be because of the relatively greater allocation of patients from the low-risk group to the HBCR group. Nevertheless, the increased moderate-intensity exercise duration in both groups suggests that CR program has a positive effect on improving exercise habits.
However, we did not find any significant changes in the high-intensity exercise duration and showed increasing results in the number of patients smoking. Patients who were smoking or started smoking cessation were given feedback to quit smoking every 3 months at an outpatient visit, but quitting smoking was not easy and feedback about once every 3 months was not enough to get them to quit smoking, which is why this result appeared. Previous review studies have suggested that smoking is related to CAD severity and the location of the damaged artery in the heart [20]. Cameron et al. [21] reported that cigarette smoking cessation was associated with reduced postoperative angina. Therefore, we believe that our CR program will require a new approach for more reliable management that encourages high-intensity exercise and patients to quit smoking.
Third, in the case of the METsmax values representing CRF levels, both groups showed significantly improved results. Although the METsmax values at baseline in the two groups were different, one of the main findings of this study was that METsmax values increased in both groups and this finding is consistent with that of a previous study [22]. However, the rate of change from baseline was significantly higher in the CBCR group at 3 months. Aguiar Rosa et al. [23] reported that patients with lower baseline CRF levels presented more significant improvements in functional capacity after CR. Since METsmax were higher in the HBCR group at baseline, this finding could be attributed to the different baseline METsmax values between the two groups at baseline. However at 6 months, no significant differences were found between the two groups. The METsmax values in the CBCR group were lower at 6 months than at 3 months, although the difference was not statistically significant. This is believed to be because the CBCR group started exercising at home after 4 months of exercise at the center. Therefore, for patients transitioning from CBCR to HBCR, strategies such as smartphone-based CR are needed for long-term management [24].
This study had several limitations. First, the main objective of this study, which was the investigation of lifestyle changes, was conducted using a questionnaire survey completed subjectively by patients. Therefore, the self-reported measures of diet, exercise duration, and psychological status may have been biased because the participants could have filled out the survey by exaggerating or understating information about their lifestyles. Second, this was a retrospective cohort study. Although meta-analysis had shown that CR training can effectively improve the patient’s cardiac function indicators and self-care ability [25], this study lacks the necessary controls to identify the effect of CR. There is no clear evidence that a reduction or increase in multiple markers is solely attributable to CR. Third, there is a selection bias arising from the non-randomization process at the point of treatment selection. This resulted in statistically significant differences in baseline characteristics such as sex, diagnosis, and LVEF levels. Fourth, there is another selection bias due to the fact that only those who completed the 6-month follow-up were analyzed, and that patients with incomplete EMRs were excluded. Fifth, the study period was short, the sample size was relatively small, and the study was conducted at a single center. Therefore, it is difficult to generalize the study results, and further studies on long-term results are needed. Nevertheless, this is meaningful because it is the first study on whether lifestyle modifications are successful in CR programs. In addition, unlike the conventional CBCR, it was confirmed that the HBCR program requires self-management, but risk factors management can be sufficiently implemented through appropriate education.
In summary, whether patients do CR at home or in the center, lifestyle can be effectively modified regardless of the type of CR. Therefore, patients should participate in any form of CR to improve CRF levels and prevent heart diseases recurrences. Furthermore, in order to effectively manage the lifestyle at home for a long period of time, additional rehabilitation strategies that can anticipate the long-term effects of CR, such as smartphone-based CR, are also needed. Additionally, more aggressive strategies are required to prevent smoking.
REFERENCES
2. McMahon SR, Ades PA, Thompson PD. The role of cardiac rehabilitation in patients with heart disease. Trends Cardiovasc Med. 2017; 27:420–5.

3. Ramachandran HJ, Jiang Y, Tam WWS, Yeo TJ, Wang W. Effectiveness of home-based cardiac telerehabilitation as an alternative to Phase 2 cardiac rehabilitation of coronary heart disease: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022; 29:1017–43.

4. Ruano-Ravina A, Pena-Gil C, Abu-Assi E, Raposeiras S, van 't Hof A, Meindersma E, et al. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int J Cardiol. 2016; 223:436–43.

5. Ades PA, Keteyian SJ, Wright JS, Hamm LF, Lui K, Newlin K, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts cardiac rehabilitation collaborative. Mayo Clin Proc. 2017; 92:234–42.

6. Taylor RS, Dalal H, Jolly K, Zawada A, Dean SG, Cowie A, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev. 2015; (8):CD007130.

7. Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev. 2017; 6:CD007130.

8. Chu DJ, Al Rifai M, Virani SS, Brawner CA, Nasir K, Al-Mallah MH. The relationship between cardiorespiratory fitness, cardiovascular risk factors and atherosclerosis. Atherosclerosis. 2020; 304:44–52.

9. Bhat AG, Farah M, Szalai H, Lagu T, Lindenauer PK, Visintainer P, et al. Evaluation of the American Association of Cardiovascular and Pulmonary Rehabilitation exercise risk stratification classification tool without exercise testing. J Cardiopulm Rehabil Prev. 2021; 41:257–63.

10. Trabulo M, Mendes M, Mesquita A, Seabra-Gomes R. [Does the modified Bruce protocol induce physiological stress equal to that of the Bruce protocol?]. Rev Port Cardiol. 1994; 13:753–6. 753-60; 735-6. Portuguese.
11. National Health Insurance Service. Health checkup questionnaire [Internet]. Wonju: National Health Insurance Service;[cited 2023 Jun 21]. Available from: https://www.nhis.or.kr/nhis/healthin/wbhaca04500m01.do.
12. Chindhy S, Taub PR, Lavie CJ, Shen J. Current challenges in cardiac rehabilitation: strategies to overcome social factors and attendance barriers. Expert Rev Cardiovasc Ther. 2020; 18:777–89.

13. Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998;280:2001-7. Erratum in: JAMA. 1999; 281:1380.
14. Schwaab B, Zeymer U, Jannowitz C, Pittrow D, Gitt A. Improvement of low-density lipoprotein cholesterol target achievement rates through cardiac rehabilitation for patients after ST elevation myocardial infarction or non-ST elevation myocardial infarction in Germany: results of the PATIENT CARE registry. Eur J Prev Cardiol. 2019; 26:249–58.

15. Wilson PW. Cardiac rehabilitation and getting to lipid goals. J Cardiopulm Rehabil. 2005; 25:264–5.

16. Snow R, LaLonde M, Hindman L, Falko J, Caulin-Glaser T. Independent effect of cardiac rehabilitation on lipids in coronary artery disease. J Cardiopulm Rehabil. 2005; 25:257–61. quiz 262-3.

17. Athyros VG, Liberopoulos EN, Mikhailidis DP, Papageorgiou AA, Ganotakis ES, Tziomalos K, et al. Association of drinking pattern and alcohol beverage type with the prevalence of metabolic syndrome, diabetes, coronary heart disease, stroke, and peripheral arterial disease in a Mediterranean cohort. Angiology. 2007; 58:689–97.

18. Huntgeburth M, Ten Freyhaus H, Rosenkranz S. Alcohol consumption and hypertension. Curr Hypertens Rep. 2005; 7:180–5.

20. Salehi N, Janjani P, Tadbiri H, Rozbahani M, Jalilian M. Effect of cigarette smoking on coronary arteries and pattern and severity of coronary artery disease: a review. J Int Med Res. 2021; 49:3000605211059893.

21. Cameron AA, Davis KB, Rogers WJ. Recurrence of angina after coronary artery bypass surgery: predictors and prognosis (CASS Registry). Coronary Artery Surgery Study. J Am Coll Cardiol. 1995; 26:895–9.
22. Kim C, Choi HE, Jang JH, Song JH, Kim BO. Do patients maintain proper long-term cardiopulmonary fitness levels after cardiac rehabilitation? A retrospective study using medical records. Ann Rehabil Med. 2021; 45:150–9.

23. Aguiar Rosa S, Abreu A, Marques Soares R, Rio P, Filipe C, Rodrigues I, et al. Cardiac rehabilitation after acute coronary syndrome: do all patients derive the same benefit? Rev Port Cardiol. 2017; 36:169–76.

Fig. 1.
Flow diagram indicating progress of patients through the study. CR, cardiac rehabilitation; EMRs, electronic medical records.
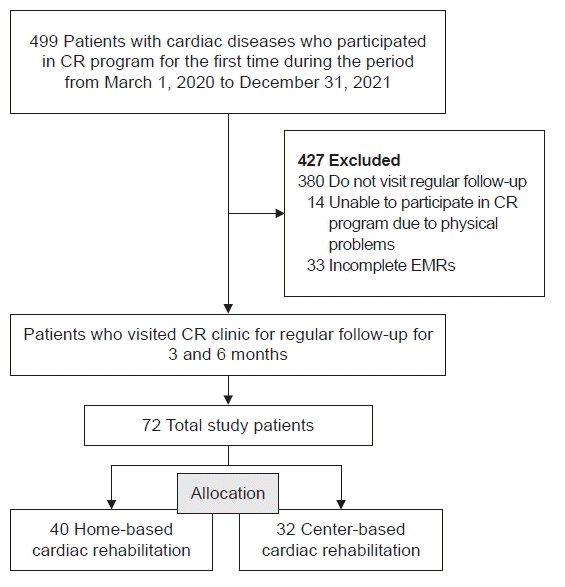
Fig. 2.
Six months’ trend of data between the HBCR (dotted line) and CBCR (solid line) groups. (A) BMI (kg/m2), (B) HbA1c (%), (C) LDL (mg/dL), (D) HDL (mg/dL), (E) alcohol habits (day/month), (F) psychological status (1-calm, 2-mild unstable, 3-very unstable), (G) diet habits of eating high-cholesterol food (1-often, 2-sometimes, 3-not), (H) METsmax (mL/kg/min), (I) high-intensity exercise habits (min/week), and (J) moderate-intensity exercise habits (min/week). CBCR, center-based cardiac rehabilitation; HBCR, home-based cardiac rehabilitation; BMI, body mass index; HbA1c, glycated hemoglobin; LDL, low-density lipoprotein; HDL, high-density lipoprotein; METsmax, maximal metabolic equivalents. a)p<0.05, significantly different at 3 and 6 months from the baseline value in both groups, no significant difference between 3 and 6 months in both groups. b)p<0.05, significantly different between the two groups at baseline, no significant difference between the two groups at 3 and 6 months.
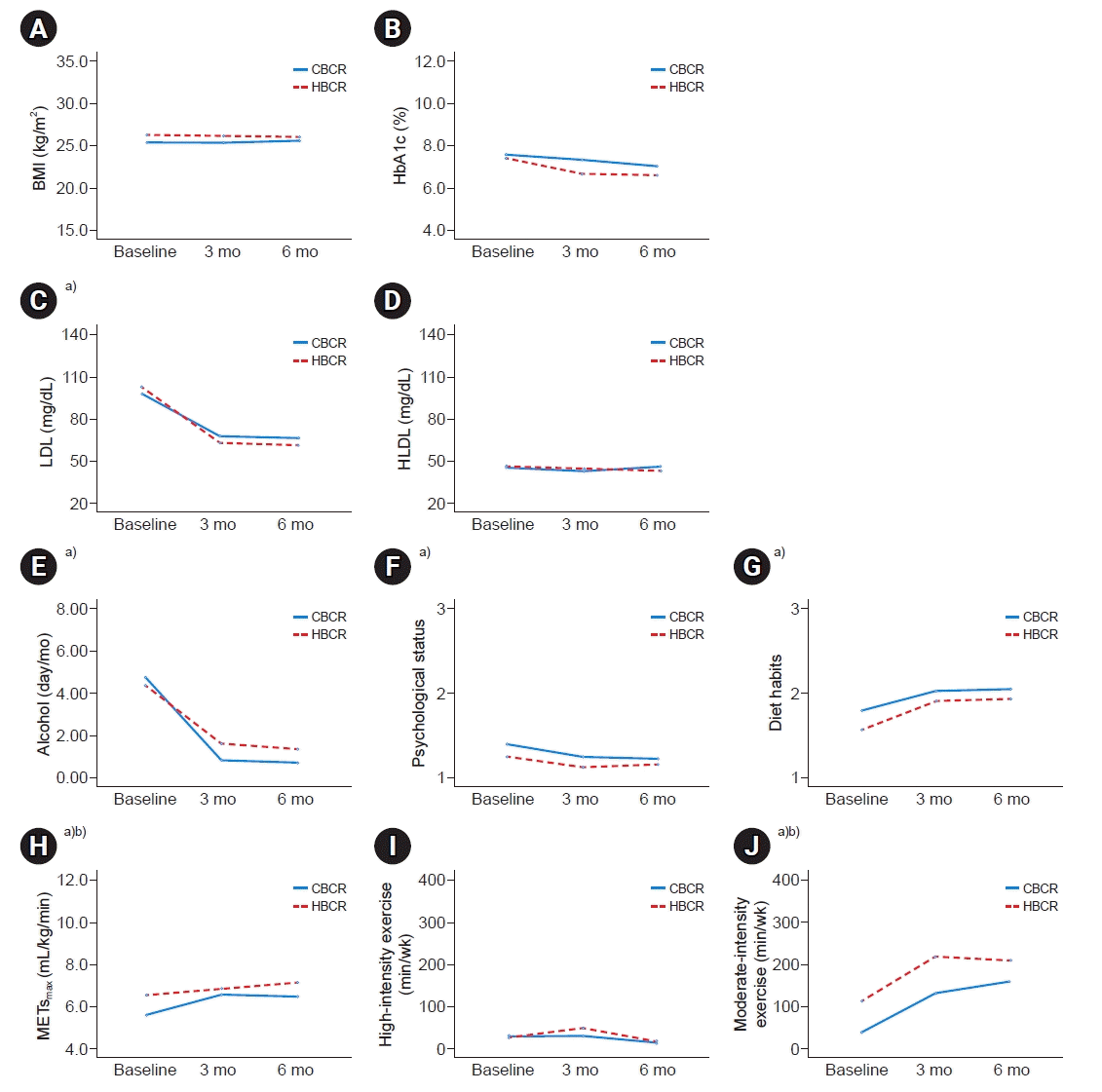
Table 1.
Baseline characteristics of patients in the HBCR and CBCR groups
| Characteristic | HBCR (n=32) | CBCR (n=40) | p-value |
|---|---|---|---|
| Age (yr) | 58.7±10.4 | 59.7±11.7 | 0.712 |
| Sex, male:female | 31:1 | 27:13 | <0.001* |
| Left ventricle ejection fraction (%) | 56.0±8.0 | 46.9±12.8 | <0.001* |
| Smoking history | |||
| Never | 8 (25.0) | 19 (47.5) | 0.085 |
| Ex-smoker | 19 (59.4) | 19 (47.5) | 0.085 |
| Current | 5 (15.6) | 2 (5.0) | 0.085 |
| Cardiac diagnosis | |||
| Stable angina | 4 (12.5) | 1 (2.5) | 0.128 |
| Unstable angina | 9 (28.1) | 4 (10.0) | 0.059 |
| Non-STEMI | 8 (25.0) | 8 (20.0) | 0.618 |
| STEMI | 7 (21.9) | 19 (47.5) | 0.022* |
| Others | 4 (12.5) | 8 (20.0) | 0.394 |
| Comorbidity | |||
| Stroke | 1 (3.1) | 2 (5.0) | 0.821 |
| Hypertension | 18 (56.3) | 23 (57.5) | 0.917 |
| Diabetes mellitus | 12 (37.5) | 17 (42.5) | 0.673 |
| Dyslipidemia | 8 (25.0) | 9 (22.5) | 0.807 |
| Chronic kidney disease | 0 (0.0) | 1 (2.5) | 0.375 |
| Others | 5 (15.6) | 11 (27.5) | 0.224 |
| None | 1 (3.1) | 1 (2.5) | 0.873 |
| Family history of cardiac disease | |||
| Yes | 10 (31.3) | 11 (27.5) | 0.732 |
| Intervention or operation | |||
| PCI | 27 (84.3) | 30 (75.0) | 0.337 |
| Coronary artery bypass graft | 0 (0.0) | 2 (5.0) | 0.160 |
| Medication | 2 (6.3) | 4 (10.0) | 0.574 |
| Others | 3 (9.4) | 4 (10.0) | 0.930 |
Table 2.
Comparison of 6-month trend data between the HBCR and CBCR groups
| Lifestyle and laboratory results |
HBCR (n=32) |
CBCR (n=40) | ||||
|---|---|---|---|---|---|---|
| Baseline | 3 mo | 6 mo | Baseline | 3 mo | 6 mo | |
| Body mass index (kg/m2) | 26.3±0.6 | 26.1±0.6 | 26.0±0.6 | 25.4±0.6 | 25.3±0.5 | 25.5±0.6 |
| HbA1c (%) | 7.3±0.7 | 6.6±0.6 | 6.5±0.4 | 7.5±0.6 | 7.3±0.5 | 7.0±0.4 |
| HDL (mg/dL) | 45.4±3.3 | 43.9±2.8 | 42.5±3.7 | 45.0±2.5 | 42.4±2.1 | 45.8±2.8 |
| LDL (mg/dL) | 102.4±11.3 | 62.6±6.4d) | 60.6±5.5d) | 97.0±8.6 | 67.1±4.8d) | 65.8±4.2d) |
| METsmax (mL/kg/min) | 6.6±0.3c) | 6.9±0.3d) | 7.1±0.3d) | 5.6±0.3c) | 6.6±0.3d) | 6.5±0.3d) |
| Exercise habits (min/wk) | ||||||
| High | 26.3±20.5 | 52.8±22.5 | 17.8±8.5 | 30.5±18.4 | 33.6±20.9 | 16.6±7.6 |
| Mod | 109.7±24.8c) | 219.7±30.3d) | 210.6±29.2d) | 38.1±22.2c) | 132.9±27.1d) | 159.9±26.1d) |
| Alcohol frequency (day/mo) | 4.4±1.7 | 1.6±0.5d) | 1.4±0.4d) | 4.8±1.5 | 0.8±0.4d) | 0.7±0.4d) |
| Diet habitsa) | 1.6±0.1 | 1.9±0.1d) | 1.9±0.1d) | 1.8±0.1 | 2.0±0.1d) | 2.1±0.1d) |
| Psychological statusb) | 1.3±0.1 | 1.1±0.1d) | 1.2±0.1d) | 1.4±0.1 | 1.3±0.1d) | 1.2±0.1d) |
| Current smokers (no.) | 5 | 6 | 8 | 2 | 2 | 3 |
Table 3.
Comparison of the rate of METsmax increase from baseline
|
3 mo |
6 mo |
|||
|---|---|---|---|---|
| HBCR | CBCR | HBCR | CBCR | |
| Changes of METsmax from baseline | 0.36±0.92 | 0.95±1.02 | 0.58±0.93 | 0.84±0.95 |
| p=0.014* | p=0.255** | |||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download