Abstract
Background
This study aimed to determine the predictive power of the Full Outline of Unresponsiveness (FOUR) score and the Glasgow Coma Scale Pupil (GCS-P) score in determining outcomes for traumatic brain injury (TBI) patients. The Glasgow Outcome Scale (GOS) was used to evaluate patients at 1 month and 6 months after the injury.
Methods
We conducted a 15-month prospective observational study. It included 50 TBI patients admitted to the ICU who met our inclusion criteria. We used Pearson's correlation coefficient to relate coma scales and outcome measures. The predictive value of these scales was determined using the receiver operating characteristic (ROC) curve, calculating the area under the curve with a 99% confidence interval. All hypotheses were two-tailed, and significance was defined as P<0.01.
Results
In the present study, the GCS-P and FOUR scores among all patients on admission as well as in the subset of patients who were mechanically ventilated were statistically significant and strongly correlated with patient outcomes. The correlation coefficient of the GCS score compared to GCS-P and FOUR scores was higher and statistically significant. The areas under the ROC curve for the GCS, GCS-P, and FOUR scores and the number of computed tomography abnormalities were 0.912, 0.905, 0.937, and 0.324, respectively.
Road traffic injuries are a global phenomenon, causing an annual 1.35 million deaths and resulting in many traumatic brain injuries (TBIs) [1]. The most common cause of death in children <15 years of age and young adults <45 years of age is TBI [2]. In addition to its high mortality rate, TBI is an important cause of severe morbidity among sufferers [3].
Factors that predict poor outcome in a case of TBI include a Glasgow Coma Scale (GCS) score <8 points, pupillary responsiveness to light, systolic hypotension, concomitant systemic injury, and computed tomography (CT) imaging findings suggestive of hemorrhage [4]. If assessed properly and treated congruously, mild TBI cases (GCS score, 13–15 points) have a good prognosis, and the overall mortality in this group is around 0.1% [5]. Patients with moderate to severe TBI (GCS <13 points) have a much poorer prognosis, with an approximate mortality rate of 30%. The mortality rate of those with severe TBI alone (GCS score ≤8 points) may be as high as 50% [6]. Numerous prognostic indicators like hypotension and hypoxia have been identified to predict death and functional outcome status following TBI [3]. Age is also an independent predictor of functional outcome status and mortality in patients with TBI [7,8].
There are many limitations to the GCS. Local eye trauma and swelling can affect eye responses, and patients who have an endotracheal tube cannot be assessed for verbal response component of GCS [5,6]. Only sparse data are available to suggest that additional examination of pupillary responses has any effect on the overall predictive accuracy of GCS [9-11]. In 2005, Wijdicks and colleagues developed and validated a new coma scale, the Full Outline of Unresponsiveness (FOUR) score, as a suggested replacement for GCS [12]. This tool provides auxiliary information about the functioning of the brainstem and the respiratory drive. It is still, however, unclear which among these two scoring systems has a better predictive value for mortality [12]. Bryan Jennet and Michael Bond published the Glasgow Outcome Scale (GOS) in 1975, 1 year after the GCS was published. This tool is used to assess the patient’s longer-term neurological outcome, functional status, and ability to return to work [13].
The current study aimed to assess the performance of FOUR and Glasgow Coma Scale Pupil (GCS-P) scores to predict the GOS of patients with TBI at the time of hospital discharge or at 1 month and 6 months of follow-up. It also analyzed prediction of GOS by the GCS-P score, age, and CT findings of patients with TBI.
This study was designed as a prospective observational study. It was performed in the critical care department at a tertiary care university hospital in India. Consecutive patients seen from July 2020 and September 2021 were included in the study. This study was approved by the Ethics Committee (No. BVDUMC/IEC/13, Dated:05/07/2020) and a waiver of written informed consent was obtained as the study was prospective, observational, and non-interventional with data collected anonymously. This study is registered with the Clinical Trials Registry–India (CTRI; registration no. CTRI/2020/09/027837).
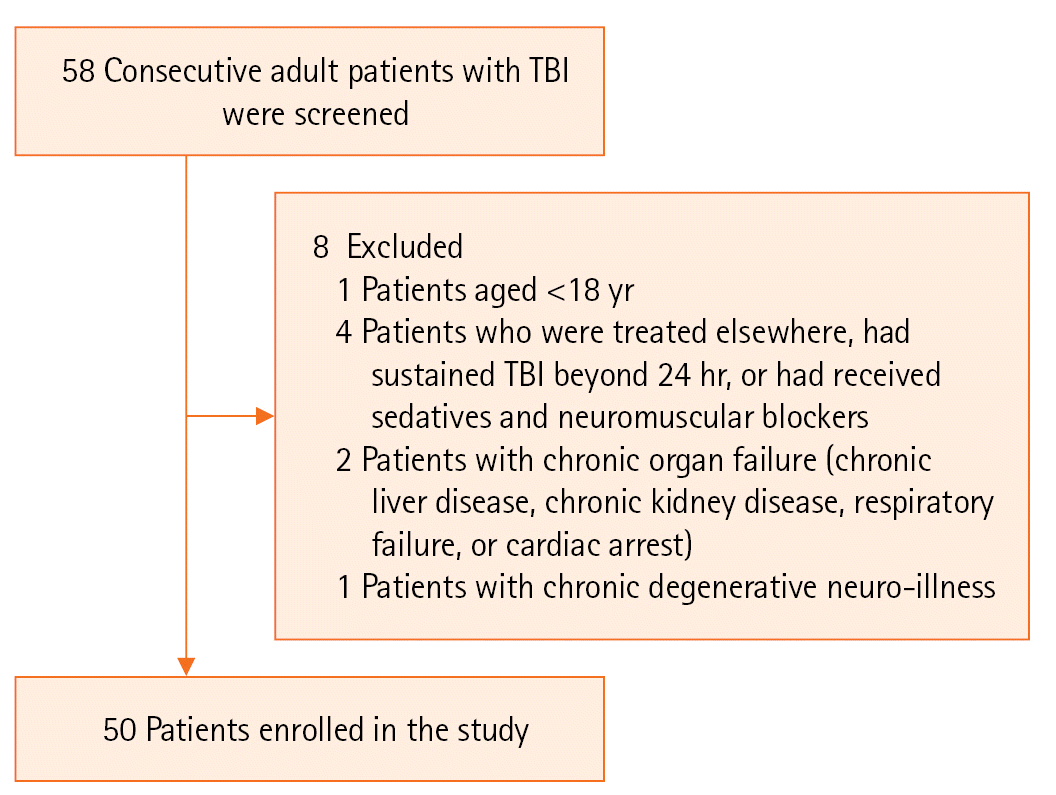
Adult patients ≥18 years of age admitted within 24 hours of TBI were included in this study. The inclusion and exclusion criteria are indicated in Figure 1. On admission, history; demographic details; and GCS, GCS-P, and FOUR scores were recorded on the data-collection sheet. Hemodynamic parameters and the presence of hypoxia, hypotension, and concomitant injuries were noted. Admission CT findings, need for and duration of mechanical ventilation, need for surgery, hospital length of stay, and need for tracheostomy were also recorded.
The Pupil Reactivity Score (PRS) summarizes information about loss of pupil reactivity to light and is calculated as follows: both pupils unreactive to light, 2 points; one pupil unreactive to light, 1 point; or both pupils reactive to light, 0 points. Then, GCS-P=GCS–PRS. The verbal score for GCS in an intubated patient is calculated as follows: able to talk, 5 points; questionable capacity to talk, 3 points; unresponsive, 1 point.
Eye response, motor response, brainstem reflexes, and respiration are all covered by the FOUR score, which is calculated as follows. Four points are awarded if the eyelids are open or opened, tracking, or blinking on command; the patient can make a thumbs-up, fist, or peace sign; pupil and corneal reflexes are present; and the patient is not intubated, with a regular breathing pattern. Three points are awarded if: the eyelids are open but not tracking; localizing to pain; one pupil is wide and fixed; and the patient is not intubated, with a Cheyne-Stokes breathing pattern. Two points are awarded if the eyelids are closed but open to a loud voice; there is a flexion response to pain; pupil or corneal reflexes are absent; and the patient is not intubated, with irregular breathing. One point is awarded if the eyelids are closed but open to pain; there is an extended response to pain; pupil and corneal reflexes are absent; and the patient breathes above the ventilator rate. Zero points are awarded if the eyelids remain closed with pain; there is no response to pain or generalized myoclonus status; there are absent pupil, corneal, and cough reflexes; and the patient breathes at the ventilator rate or shows apnea.
GOS was assessed at the time of discharge from the hospital or at 1 month from the day of admission, whichever was later, and then again at 6 months through a telephonic survey. The GOS is rated as follows: GOS-1=dead, GOS-2=vegetative state, GOS-3=severe disability, GOS-4=moderate disability, and GOS-5=mild or no disability. The functional outcome was dichotomized (poor/unfavorable outcome versus good/favorable outcome) based on GOS at the time of discharge from the hospital or 1 month after admission, whichever came later, and at 6 months. A poor outcome was defined as GOS of 1–3 points, whereas a good outcome was defined as GOS of 4–5 points.
To record their length of stay, patients were monitored until their release from the hospital. The frequencies (percentages) in each category were calculated for the categorical variables. The near-normality of the distribution for the quantitative variables was evaluated. Mean and standard deviation values were used to summarize the variables with a normal distribution (standard deviation).
Coma scales and outcome measurements were correlated using Pearson's correlation coefficient, and the area under the receiver operating characteristic curve (AUC) and 99% confidence interval were used to determine the scales' predictive value. Every hypothesis was two-tailed, and a P<0.01 was deemed statistically significant. The SPSS ver. 22.0 (IBM Corp.) for Windows was used to statistically analyze all data.
Among the 50 patients who were enrolled in this study, the age range was 19–61 years with a mean of 42±13 years, and 74% (n=37) of participants were men (Table 1). Among the enrolled patients (n=50), the mean GCS score was 11.84±3.92, the mean GCS-P score was 11.62±4.33, and the mean FOUR score was 12.98±4.99. Among patients who presented with moderate to severe TBI (n=22), the mean GCS score was 8.05 ±2.88, the mean GCS-P score was 7.55±3.43, and the mean FOUR score was 9.14±4.31 (Table 1).
To determine the predictive value of the GCS, GCS-P, and FOUR scores and the number of CT abnormalities (intracranial hematoma, absent cisterns, and subarachnoid hemorrhage) with regard to mortality, receiver operating characteristic curve analysis was used (Figure 2), and the AUC was calculated. The respective AUCs for the GCS, GCS-P, and FOUR scores and the number of Ct abnormalities were 0.912, 0.905, 0.937, and 0.324.
In the present study, the coefficient of correlation of the GCS score compared to the GCS-P and FOUR scores among all 50 patients at admission as well as in the subset of patients who were mechanically ventilated was strongly correlated and statistically significant (Table 2). Of the 43 patients who survived to hospital discharge, 1 had died by 1 month later. The correlation coefficient of GCS score was 0.996 compared to the GCS-P score and was 0.959 for all 50 patients compared to the FOUR score. When considering the ventilated patient subgroup, there was a comparable high correlation. At 1 month after discharge, the correlation coefficients for the 39 patients with a favorable outcome (GOS=4–5 points) were 0.996 for the GCS vs. GCS-P score and 0.936 for the GCS vs. FOUR score, while they were 0.997 and 0.953 for 42 patients, respectively.
At 1 month after discharge, the correlation coefficients for 11 patients with poor outcomes (GOS=1–3 points) were 0.989 for the GCS vs. GCS-P score and 0.930 for the GCS vs. FOUR score, while they were 0.986 and 0.944, respectively, for eight patients. Among the 50 patients, 14 needed mechanical ventilation, 10 needed surgery, and 5 underwent tracheostomy. The mean hospital stay was 13.07 days for ventilated patients and 7.27 days for non-ventilated patients.
Reported in 1974 by Teasdale and Jennet, the GCS remains the gold-standard method of assessing neurological status in patients with TBI [5]. However, researchers have since focused on the development of more accurate tools, which include the FOUR score and the GCS-P score. Hence, this study was conducted to compare FOUR and GCS-P scores in patients with TBI to predict their outcomes in the intensive care unit (ICU).
Our study was performed in patients aged ≥18 years admitted to the ICU with TBI. Some studies performed by various researchers such as Baratloo et al. [14], Furman et al. [15], Ghelichkhani et al. [16], Gorji et al. [17], Hossein et al. [18], Jalali and Rezaei [19], Kafle et al. [20], McNett et al. [21], Nair et al. [22], Nyam et al. [23], Okasha et al. [24], Sadaka et al. [25], Saika et al. [26], and Sepahvand et al. [27] exclusively enrolled TBI patients, whereas other studies by Bayraktar et al. [28], Bruno et al. [29], Eken et al. [30], Gujjar et al. [31], Khanal et al. [32], Ramazani and Hosseini [33], Wolf et al. [34], and Temiz et al. [35] included subjects with medical and/or neurological conditions like sepsis, stroke, brain tumors, or comatose states.
Our study sample consisted mainly of young men with a mean age of 42.08 years, and 94% of them were <60 years of age. Our findings are consistent with those reported by Jalali and Rezaei [19], in that the mean age of their sample was 41±18 years, and 77.9% of them were men. Similarly, other studies by Hossein et al. [18], Kafle et al. [20], Saika et al. [26], and Temiz et al. [35] reported relatively lower mean ages of 34±2, 39±18, 38±16, and 47±20 years, respectively. In contrast, in their study performed at Selçuk University, Bayraktar et al. [28] enrolled 79 patients admitted to the ICU whose mean age was 53±14 years, which is high compared to our findings.
In the present study of 50 samples, the various comorbidities reported were diabetes (14%), hypertension (4%), and ischemic heart disease (2%), and a few patients had multiple comorbidities like diabetes and hypertension (4%) or diabetes, hypertension, and IHD (2%). A diagnostic accuracy study comparing GCS and FOUR scores in predicting mortality in trauma patients by Ghelichkhani et al. [16] at Tehran University of Medical Sciences in Iran reported comorbidity trends consistent with our study findings.
In the present study, the mean GCS score reported on admission was 11.84±3.92 points, while the mean FOUR score was 12.98±4.99 points and the mean GCS-P score was 11.62±4.33 points. Also, the mean scores of GOS at 1 month and 6 months after discharge were 4.2±1.46 and 4.32±1.48 points, respectively. These findings are consistent with those of the study by Kafle et al. [20] of 122 head injury patients attending Tribhuvan University Teaching Hospital in Nepal, which reported a mean GCS score of 10.43±2.5 points and a mean FOUR score of 12.15±3.15 points.
Existing studies have shown that AUCs for prediction of mortality for GCS and FOUR scores vary between 0.72 and 0.96 in published studies, and the scores correlated well with each other (Table 3). Studies performed in different settings and differences in the skill level of health care workers in assessing comatose patients could contribute to this variation. None of the available studies to the best of our knowledge have directly compared and correlated the GCS-P and FOUR scores.
The GCS-P score adds evaluation of the pupil, which is very subjective and may not be more precise than the GCS score. A more precise assessment of the size of the pupil by pupillometer may help to predict the outcome more accurately than the GCS-P score alone. Automated infrared pupillometry using a handheld device will allow more objective and quantitative measurement of pupillary function compared to a visual inspection. Further assessment of brainstem reflexes and respiratory pattern, as seen in the FOUR score, adds complexity at bedside.
The strengths of this study include supplementation of limited studies comparing the GCS, GCS-P, and FOUR scores. Hence, it is a unique study that investigates the correlation between GCS, GCS-P, and FOUR scores at admission with the GOS at both 1 month and 6 months after discharge. The follow-up was performed meticulously, and there was no patient lost to follow-up. In a sample of patients with moderate-to-severe TBI, the correlations between the GCS, GCS-P, and FOUR scores and the GOS were examined individually.
The limitations of the study are as follows. Our sample size was only 50 patients, as cases of road traffic accidents with TBI were limited in number due to the coronavirus disease 2019 pandemic, and patients who were treated elsewhere were excluded from the study along with those with chronic organ failures, chronic degenerative neuro-illness, or who had already received sedative drugs. Despite the lack of significant difference in the correlation between the scores of patients in our study with moderate-to-severe TBI, a larger investigation focused only on such a subset of patients is necessary. Among the cohort of patients who visited our hospital, few had moderate-to-severe TBI during the study period, and enrolling more patients with this condition might change the end result. Admission GCS scores may vary depending on how quickly the patients present to the hospital. Assessing GCS, GCS-P, and FOUR scores just prior to intubation as well as at the time of admission may ensure a more accurate correlation with the final outcome and should be studied further. Finally, the use of a pupillometer might have improved the accuracy of our measurements.
In this prospective observational study, the GCS-P and FOUR scores did not show any significant difference in the prediction of outcomes among patients with mild to moderate TBI. We also conclude that the gold-standard GCS score compared to the GCS-P and FOUR scores shows the best correlation with outcome in patients with mild to moderate TBI. It remains to be seen whether the GCS-P score or the FOUR score will perform better in a cohort of severe TBI patients and when an objective assessment of the pupils is conducted with a pupillometer.
▪ This study assessed the ability of the Glasgow Coma Scale (GCS), Glasgow Coma Scale Pupil (GCS-P), and Full Outline of Unresponsiveness (FOUR) scores to predict outcomes among patients with traumatic brain injury in the intensive care unit.
▪ The GCS score, which is most commonly calculated at bedside, had the best correlation, although all three scores were similar..
REFERENCES
1. World Health Organization. Road traffic injuries [Internet]. World Health Organization; 2021 [cited 2023 Apr 4]. Available from: https://www.who.int/news-room/fact-sheets/detail/road-traffic-injuries.
3. Emami P, Czorlich P, Fritzsche FS, Westphal M, Rueger JM, Lefering R, et al. Impact of Glasgow coma scale score and pupil parameters on mortality rate and outcome in pediatric and adult severe traumatic brain injury: a retrospective, multicenter cohort study. J Neurosurg. 2017; 126:760–7.

4. Klauber MR, Marshall LF, Luerssen TG, Frankowski R, Tabaddor K, Eisenberg HM. Determinants of head injury mortality: importance of the low risk patient. Neurosurgery. 1989; 24:31–6.

5. Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. Br J Anaesth. 2007; 99:18–31.

6. Rabiu TB. Revisiting the eye opening response of the Glasgow coma scale. Indian J Crit Care Med. 2011; 15:58–9.

7. Mosenthal AC, Lavery RF, Addis M, Kaul S, Ross S, Marburger R, et al. Isolated traumatic brain injury: age is an independent predictor of mortality and early outcome. J Trauma. 2002; 52:907–11.

8. Lieberman JD, Pasquale MD, Garcia R, Cipolle MD, Mark Li P, Wasser TE. Use of admission Glasgow coma score, pupil size, and pupil reactivity to determine outcome for trauma patients. J Trauma. 2003; 55:437–43.

9. Hoffmann M, Lehmann W, Rueger JM, Lefering R, Trauma Registry of the German Society for Trauma Surgery. Introduction of a novel trauma score. J Trauma Acute Care Surg. 2012; 73:1607–13.

10. Healey C, Osler TM, Rogers FB, Healey MA, Glance LG, Kilgo PD, et al. Improving the Glasgow coma scale score: motor score alone is a better predictor. J Trauma. 2003; 54:671–80.

11. Hoffmann M, Lefering R, Rueger JM, Kolb JP, Izbicki JR, Ruecker AH, et al. Pupil evaluation in addition to Glasgow coma scale components in prediction of traumatic brain injury and mortality. Br J Surg . 2012; 99 Suppl 1:122–30.

12. Foo CC, Loan JJ, Brennan PM. The relationship of the FOUR score to patient outcome: a systematic review. J Neurotrauma. 2019; 36:2469–83.

13. Teasdale GM, Pettigrew LE, Wilson JT, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow outcome scale. J Neurotrauma. 1998; 15:587–97.

14. Baratloo A, Shokravi M, Safari S, Aziz AK. Predictive value of Glasgow coma score and full outline of unresponsiveness score on the outcome of multiple trauma patients. Arch Iran Med. 2016; 19:215–20.
15. Furman MR, Gorenjak M, Ravnik J. FOUR score versus GCS in patients with traumatic brain injury in the prehospital setting. Research Square [Preprint]. 2020 [cited 2023 April 4]. Available from: https://doi.org/10.21203/rs.3.rs-18036/v4.
16. Ghelichkhani P, Esmaeili M, Hosseini M, Seylani K. Glasgow coma scale and FOUR score in predicting the mortality of trauma patients; a diagnostic accuracy study. Emerg (Tehran). 2018; 6:e42.
17. Gorji MA, Hoseini SH, Gholipur A, Mohammadpur RA. A comparison of the diagnostic power of the full outline of unresponsiveness scale and the Glasgow coma scale in the discharge outcome prediction of patients with traumatic brain injury admitted to the intensive care unit. Saudi J Anaesth. 2014; 8:193–7.

18. Hosseini SH, Ayyasi M, Akbari H, Heidari Gorji MA. Comparison of Glasgow coma scale, full outline of unresponsiveness and acute physiology and chronic health evaluation in prediction of mortality rate among patients with traumatic brain injury admitted to intensive care unit. Anesth Pain Med. 2016; 7:e33653.

19. Jalali R, Rezaei M. A comparison of the Glasgow coma scale score with full outline of unresponsiveness scale to predict patients’ traumatic brain injury outcomes in intensive care units. Crit Care Res Pract. 2014; 2014:289803.

20. Kafle P, Sharma MR, Shilpakar SK, Sedain G, Pradhanang A, Shrestha RK, et al. Can full outline of unresponsiveness score (FOUR) replace glasgow coma scale (GCS) in head injury?: validation at teritiary care centre in Nepal. J Univ Coll Med Sci. 2018; 6:32–9.

21. McNett M, Amato S, Gianakis A, Grimm D, Philippbar SA, Belle J, et al. The FOUR score and GCS as predictors of outcome after traumatic brain injury. Neurocrit Care. 2014; 21:52–7.

22. Nair SS, Surendran A, Prabhakar RB, Chisthi MM. Comparison between FOUR score and GCS in assessing patients with traumatic head injury: a tertiary centre study. Int Surg J. 2017; 4:656–62.

23. Nyam TE, Ao KH, Hung SY, Shen ML, Yu TC, Kuo JR. FOUR score predicts early outcome in patients after traumatic brain injury. Neurocrit Care. 2017; 26:225–31.

24. Okasha AS, Fayed AM, Saleh AS. The FOUR score predicts mortality, endotracheal intubation and ICU length of stay after traumatic brain injury. Neurocrit Care. 2014; 21:496–504.

25. Sadaka F, Patel D, Lakshmanan R. The FOUR score predicts outcome in patients after traumatic brain injury. Neurocrit Care. 2012; 16:95–101.

26. Saika A, Bansal S, Philip M, Devi BI, Shukla DP. Prognostic value of FOUR and GCS scores in determining mortality in patients with traumatic brain injury. Acta Neurochir (Wien). 2015; 157:1323–8.

27. Sepahvand E, Jalali R, Mirzaei M, Ebrahimzadeh F, Ahmadi M, Amraii E. Glasgow coma scale versus full outline of unresponsiveness scale for prediction of outcomes in patients with traumatic brain injury in the intensive care unit. Turk Neurosurg. 2016; 26:720–4.
28. Bayraktar YS, Sahinoglu M, Cicekci F, Kara I, Karabagli H, Duman A, et al. Comparison of Glasgow coma scale and full outline of unresponsiveness (Four) score: a prospective study. Turk Neurosurg. 2019; 29:285–8.

29. Bruno MA, Ledoux D, Lambermont B, Damas F, Schnakers C, Vanhaudenhuyse A, et al. Comparison of the full outline of unresponsiveness and Glasgow Liege Scale/Glasgow Coma Scale in an intensive care unit population. Neurocrit Care. 2011; 15:447–53.

30. Eken C, Kartal M, Bacanli A, Eray O. Comparison of the full outline of unresponsiveness score coma scale and the Glasgow coma scale in an emergency setting population. Eur J Emerg Med. 2009; 16:29–36.

31. Gujjar AR, Jacob PC, Nandhagopal R, Ganguly SS, Obaidy A, Al-Asmi AR. Full outline of unresponsiveness score and Glasgow coma scale in medical patients with altered sensorium: interrater reliability and relation to outcome. J Crit Care. 2013; 28:316.

32. Khanal K, Bhandari SS, Shrestha N, Acharya SP, Marhatta MN. Comparison of outcome predictions by the Glasgow coma scale and the full outline of unresponsiveness score in the neurological and neurosurgical patients in the intensive care unit. Indian J Crit Care Med. 2016; 20:473–6.

33. Ramazani J, Hosseini M. Comparison of full outline of unresponsiveness score and Glasgow coma scale in medical intensive care unit. Ann Card Anaesth. 2019; 22:143–8.

Figure 2.
Receiver operating characteristic curve of Glasgow Coma Scale (GCS), Glasgow Coma Scale Pupil (GCS-P), Full Outline of Unresponsiveness (FOUR) scores, and number of computed tomography (CT) abnormalities.
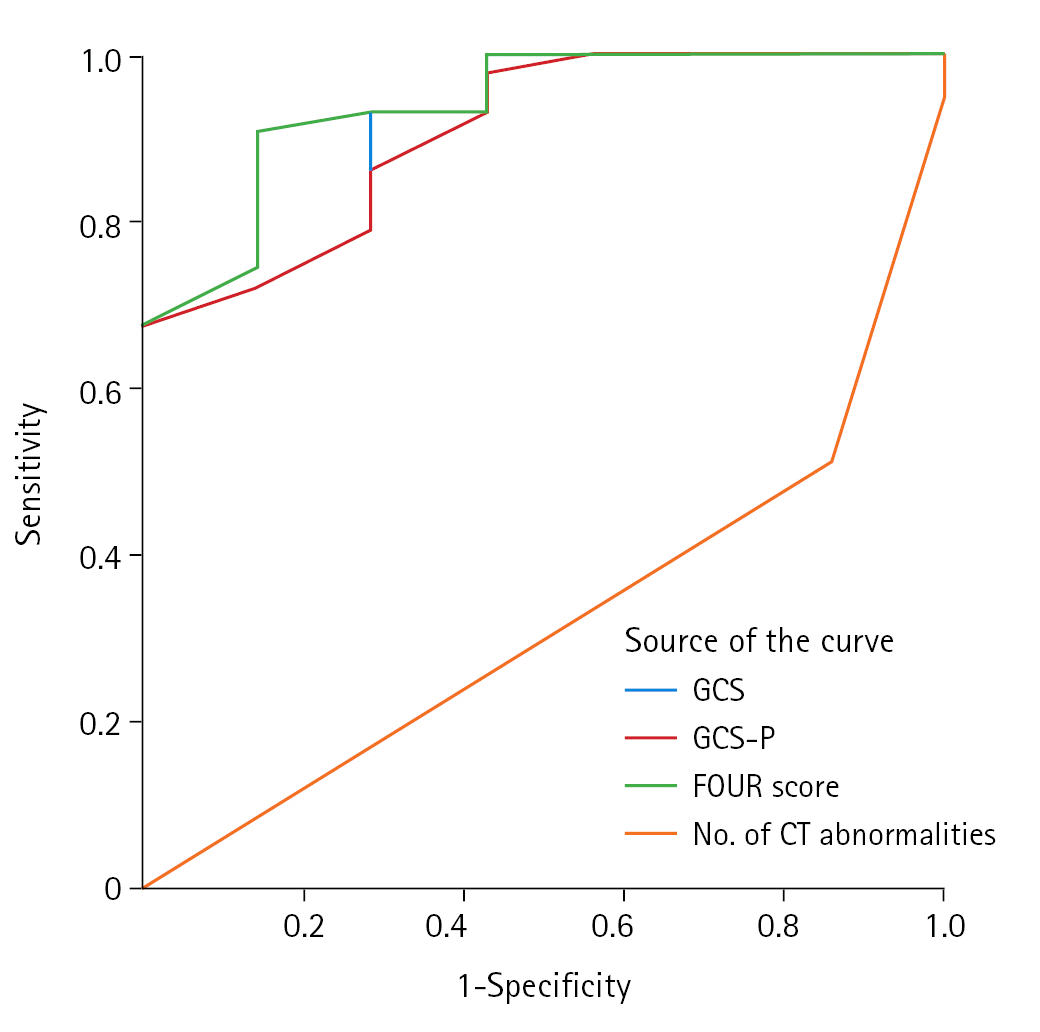
Table 1.
Patient demographic details and neurological assessment score
Table 2.
Pearson’s correlation between GCS, GCS-P, and FOUR score with the final outcomes
| Score | All patients (n=50) | Mechanically ventilated patients (n=14) |
Outcome at 1 month of discharge |
Outcome at 6 months of discharge |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Favorable (n=39) | Unfavorable (n=11) | Favorable (n=42) | Unfavorable (n=8) | |||||||||||||||
| GOS=4 and 5 | GOS=1, 2, and 3 | GOS=4 and 5 | GOS=1, 2, and 3 | |||||||||||||||
| GCS | GCS-P | FOUR score | GCS | GCS-P | FOUR score | GCS | GCS-P | FOUR score | GCS | GCS-P | FOUR score | GCS | GCS-P | FOUR score | GCS | GCS-P | FOUR score | |
| GCS | 1 | 0.996a) | 0.959a) | 1 | 0.987a) | 0.923a) | 1 | 0.996a) | 0.936a) | 1 | 0.989a) | 0.930a) | 1 | 0.997a) | 0.953a) | 1 | 0.986a) | 0.944a) |
| GCS-P | 0.996a) | 1 | 0.968a) | 0.987a) | 1 | 0.942a) | 0.996a) | 1 | 0.932a) | 0.989a) | 1 | 0.955a) | 0.997a) | 1 | 0.951a) | 0.986a) | 1 | 0.970a) |
| FOUR score | 0.959a) | 0.968a) | 1 | 0.923a) | 0.942a) | 1 | 0.936a) | 0.932a) | 1 | 0.930a) | 0.955a) | 1 | 0.953a) | 0.951a) | 1 | 0.944a) | 0.970a) | 1 |
Table 3.
Comparing the AUC values for GCS and FOUR scores in prediction of mortality
| Study |
AUC values in prediction of mortality |
|
|---|---|---|
| GCS score | FOUR score | |
| Gorji et al. [17] | 0.96 | 0.92 |
| Hossein et al. [18] | 0.96 | 0.92 |
| McNett et al. [21] | 0.93 | 0.91 |
| Baratloo et al. [14] | 0.85 | 0.86 |
| Ghelichkhani et al. [16] | 0.87 | 0.88 |
| Nyam et al. [23] | 0.74 | 0.74 |
| Kafle et al. [20] | 0.97 | 0.98 |
| Saika et al. [26] | 0.93 | 0.91 |
| Temiz et al. [35] | 0.85 | 0.85 |
| Eken et al. [30] | 0.72 | 0.77 |
| Okasha et al. [24] | 0.79 | 0.85 |
| Ramazani and Hosseini [33] | 0.82 | 0.87 |
| Sadaka et al. [25] | 0.89 | 0.93 |
| Sepahvand et al. [27] | 0.92 | 0.96 |
| This study | 0.91 | 0.93 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download