Abstract
Background
Polytrauma from road accidents is a common cause of hospital admissions and deaths, frequently leading to acute kidney injury (AKI) and impacting patient outcomes.
Methods
This retrospective, single-center study included polytrauma victims with an Injury Severity Score (ISS) >25 at a tertiary healthcare center in Dubai.
Results
The incidence of AKI in polytrauma victims is 30.5%, associated with higher Carlson comorbidity index (P=0.021) and ISS (P=0.001). Logistic regression shows a significant relationship between ISS and AKI (odds ratio [OR], 1.191; 95% confidence interval [CI], 1.150–1.233; P<0.05). The main causes of trauma-induced AKI are hemorrhagic shock (P=0.001), need for massive transfusion (P<0.001), rhabdomyolysis (P=0.001), and abdominal compartment syndrome (ACS; P<0.001). On multivariate logistic regression AKI can be predicated by higher ISS (OR, 1.08; 95% CI, 1.00–1.17; P=0.05) and low mixed venous oxygen saturation (OR, 1.13; 95% CI, 1.05–1.22; P<0.001). The development of AKI after polytrauma increases length of stay (LOS)-hospital (P=0.006), LOS-intensive care unit (ICU; P=0.003), need for mechanical ventilation (MV) (P<0.001), ventilator days (P=0.001), and mortality (P<0.001).
Dubai is one of the most progressive cities in the world. In the last two decades, it has witnessed exponential growth in terms of economics and infrastructure [1]. Dubai is a popular attraction point for expatriates to live and work because it offers high income, low taxes, and modern lifestyle [1]. Here, the incidents of road traffic accidents (RTAs) are significant due to the availability of cheap oil and high-speed vehicles, diverse population dynamics, and various driving habits [2]. Also in spite of stringent safety measures taken on infrastructure development projects, occasional cases of work-related injuries or fall accidents are seen [3]. Thus, polytrauma due to RTAs, fall accidents, or work-related injuries are not uncommon in Dubai [2,3]. Polytrauma is one of the most common causes of hospital admissions and deaths [4,5]. Kidneys are most vulnerable to dysfunction in polytrauma patients because of various trauma-related pathological factors [4,5]. The reported incidence of acute kidney injury (AKI) in polytrauma victims is 25%–40% [4-6]. Patients with old age, with comorbid conditions like hypertension, diabetes mellitus, ischemic heart disease, and with underlying renal dysfunction are highly vulnerable to developing AKI after polytrauma [7]. The usual causes of AKI in polytrauma patients are hemorrhagic/hypovolemic shock, rhabdomyolysis, abdominal compartment syndrome (ACS), use of contrast media for various radiological investigations, hypotension associated with surgery/anesthesia and sedation, use of blood and blood product, use of nephrotoxic drugs, and direct injury to kidney, ureter, and bladder [8]. The AKI has a significant impact on patient’s outcomes after polytrauma; it can increase morbidity, mortality, length of stay (LOS) in the hospital, cost of treatment, and intensive care unit (ICU) admission [8].
With this background, we planned an epidemiological study. The main purpose of this study was to determine the incidence, common causes, and predictors of AKI after polytrauma. Also, to know its impact on patient outcomes after polytrauma. Thereby, to upgrade our knowledge, preparedness, and resources to efficiently tackle this one of the vulnerable organ dysfunction counteracting during severe polytrauma.
This is a single-center, retrospective, and observational study done at tertiary health care center (THCC) in the state. The THCC, which is a 762 bedded apex trauma center in the northern part of the state, receives a major portion of polytrauma victims in the state. This study was done after approval from Institutional Scientific Research Ethics Committee (No. DSREC-04/2022_04). The written informed consent from patients was waived due to the use of retrospective de-identified data in the study.
Polytrauma victims, admitted to THCC between January 1, 2017 and December 31, 2021, were studied. Patients were selected who met all of the following criteria: (1) They were between 18 and 60 years old. (2) They were admitted within 12 hours following severe polytrauma. (3) They had Injury Severity Scores (ISS) of more than 25. (4) They underwent a major surgical procedure and was admitted to ICU. Patients who were excluded who met all of the following criteria: (1) They had ISS less than 25. (2) They were admitted 12 hours after they had polytrauma. (3) They were admitted or resuscitated in the other healthcare facility before admission to the THCC. (4) They had pre-existing renal dysfunction, or they were renal transplant recipients. (5) They had received contrast media more than one time. (6) They were with direct injuries to kidney, ureter, or bladder. (7) They were with more than 5% burns. (8) They were tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA polymerase chain reaction.
After reviewing patient’s electronic medical record (EMR), “epic hyperspace version 2022” (Epic Systems Corp.) data was collected. On a daily basis, the EMR of eligible patients were followed up from the day of admission to the day of discharge or death. The following definitions were used during data collection: (1) For comorbidity, we use Charlson Comorbidity Index (CCI) to quantify the severity of patient comorbidities [9]. (2) A polytrauma victim was defined as any patient who presents with multisystem trauma following RTA, fall from height, or work-related injuries and has an ISS score of more than 25 [10]. (3) For a hypotensive patient requiring noradrenaline support, shock was defined as a dose given that is higher than 0.1 μg/kg/min in order to keep mean blood pressure more than 65 mm Hg. (4) Serum lactate, mixed venous oxygen saturation (ScvO2), and base excess were recorded from on-admission arterial or venous blood gas. (5) Massive transfusion was defined in case of one of the situations: replacement of one entire blood volume within 24 hours, transfusion of >10 units of packed red blood cells (PRBCs) in 24 hours, transfusion of >20 units of PRBCs in 24 hours, transfusion of >4 units of PRBCs in 1 hour when on-going need is foreseeable, or replacement of 50% of total blood volume within 3 hours [11]. (6) Use of contrast media was defined as intravenous administration of 120 ml of Iohexol (Omnipaque, GE HealthCare) 350 mg/ml for computed tomography polytrauma protocol once. (7) Rhabdomyolysis was defined as an increased serum creatinine phosphokinase (CPK) level of more than 6,000 U/L. [12]. (8) A major surgical procedure was defined as definitive or damage control surgical procedure done under general anesthesia that satisfied one of the following conditions: (1) It lasted for more than 3 hours of duration. (2) It had an estimated blood loss of more than 1,000 ml. (3) It required perioperative blood transfusion of more than 2 units of PRBC [13]. (9) Nephrotoxic drugs were defined as the use of any of the following drugs: aminoglycosides, amphotericin B, angiotensin-converting enzyme (ACE) inhibitors, angiotensin-receptor blockers, or non-steroidal anti-inflammatory drugs, diuretics. (10) AKI was defined and staged according to KDIGO 2012 clinical practice definition [14]. (11) ACS was defined as an intraabdominal pressure greater than 20 mm Hg associated with new organ dysfunction or failure [15]. (12) Full renal recovery was defined as the occurrence of the patient serum creatinine returning to normal level or baseline [16]. (13) The requirement of renal replacement therapy (RRT) was defined when RRT was done for any of the following indications: volume overload, metabolic acidosis, hyperkalemia, uremia, or persistent/progressive AKI [17]. Continuous venovenous hemodiafiltration mode of RRT is available in our ICU, and that was used in all patients who required RRT.
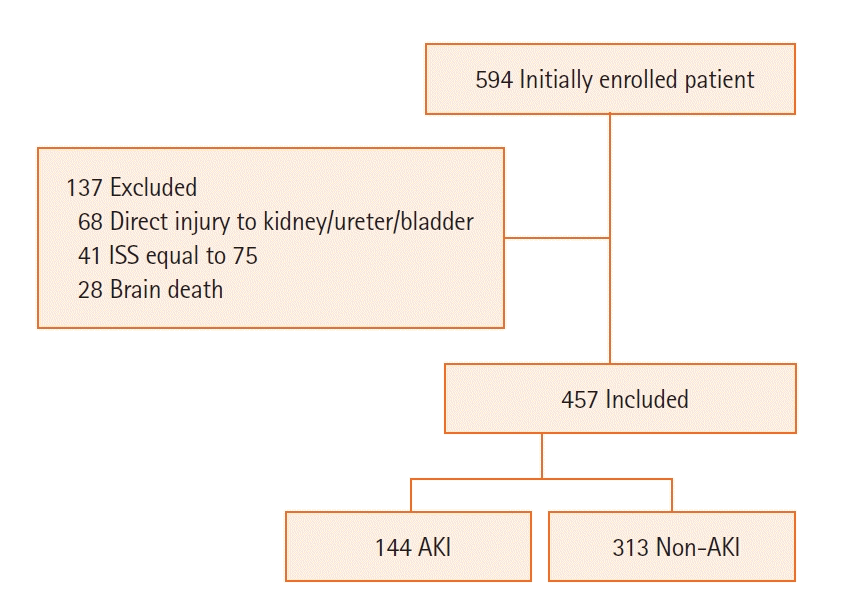
Initially, we had enrolled a total of 594 patients with ISS more than 25 in the study. But later, we excluded 137 patients. Patients excluded had direct injuries to the kidney, ureter, or bladder diagnosed on computed tomography scan (n=68), had ISS equal to 75 i.e., unsurvivable injuries (n=41), or had developed brain death during the course of management (n=28) (Figure 1). Based on the development of AKI as per KDIGO 2012 clinical practice definition, patients were divided into two groups, viz., the non-AKI group and the AKI group.
The primary objective was to measure the incidence of AKI in polytrauma patients. The secondary objective was to determine risk factors for the development of AKI and its impact on the patient’s LOS in hospital, LOS-ICU, need of mechanical ventilation, and mortality.
The data were collected in a Microsoft Excel spreadsheet and analyzed by IBM SPSS ver. 24.0 (IBM Corp.). The categorical variables were represented by frequencies and proportions and analyzed by the Pearson chi-square test. The quantitative variables with normal distribution were represented by the mean and standard deviation and analyzed by the independent samples test. The quantitative variables with skewed distribution were represented by the median and interquartile range and analyzed by the Mann-Whitney U-test. A probability value less than 0.05 (P<0.05) was considered as the point of statistical significance. Finally, to predict the AKI after polytrauma we created either a univariate or multivariate logistic regression model by using the variables with probability values less than 0.05.
The demographic characters are shown in Table 1. They were comparable in both groups. Polytrauma was more common among young, male, and expatriate population. The most common mode of polytrauma was RTA. The overall incidence of AKI in polytrauma victims was 30.5%. AKI was common in the patient who had comorbidities. The patient in the AKI group had statistically higher CCI as compared to non-AKI (0.12±0.38 vs. 0.23±0.51, P=0.020). AKI was common in patients who sustained severe injuries as indicated by higher ISS (36.0 [33.0–41.0] vs. 48.0 [37.5–48.0], P=0.001). On the logistic regression model (Figure 2), when the severity of injury (measured by the value of ISS) was compared with the occurrence of AKI. It has shown good regression between them with odds ratio (OR) 1.191 and 95% confidence interval (CI) 1.150–1.233 (P<0.05).
The causes of AKI are summarized in the Table 2. There was a significant statistical difference with regards to the presence of shock on admission (yes: 081 [25.9%] vs. 133 [92.4%], no: 232 [74.1%] vs. 11 [7.6%], P=0.001), on admission serum lactate (2.3 [1.4–4.1] vs. 6.6 [4.5–8.9], P=0.001), ScvO2 (75 [75–75] vs. 59 [55–65], P=0.001), and base excess (–4 [2–6] vs. –10 [9–14], P=0.001) between the Non-AKI group to AKI group. High serum lactate, mixed venous oxygen saturation, and base excess in the AKI group indicate that the patients of the AKI group suffered a severe shock. The need of massive transfusion was also high in the AKI group (yes: 45 [14.4%] vs. 131 [91.0%], no: 268 [85.6%] vs. 13 [9.0%], P<0.001). The rhabdomyolysis was more frequent in the AKI group as compared to the non-AKI group (yes: 61 [19.5%] vs. 057 [39.6%], no: 252 [80.5%] vs. 087 [60.4%], P=0.001). However, there was no significant difference between the maximally observed CPK level in both groups (9,306 [73–12,304] vs. 9,768 [7,221–18,775], P=0.368). None of the patients in either group received nephrotoxic drugs. The ACS was reported at a higher rate in the AKI group as compared to the non-AKI group (yes: 6 [1.9%] vs. 23 [16.0%], no: 307 [98.1%] vs. 121 [84.0%], P<0.001).
On the multivariate logistic regression model (Table 3) the main predictors of AKI after polytrauma were higher ISS (OR, 1.08; 95% CI, 1.00–1.17; P=0.05) and low SCVO2 (OR, 1.13; 95% CI, 1.05–1.22; P<0.001).
The outcome of the patient after AKI is summarized in Table 4. The LOS in the hospital and LOS-ICU in days were higher in a patient who had developed AKI (28 [16–51] vs. 37 [18–73.5], P=0.006; 12 [7–19] vs. 15 [9–23]; P=0.003). A statistically significant proportion of patients who had developed AKI were mechanically ventilated (yes: 255 (81.46%) vs. 138 (95.83%), no: 58 (8.94%) vs. 6 (4.16%), P<0.001). Also, the patients from the AKI group spent more days on a ventilator (9 [5–16] vs. 7 [12–20], P=0.001). The 28-day mortality was higher in the AKI group (8 [2.6%] vs. 25 [16.7%], P=0.001). Also, the more than 28-day mortality was higher in the AKI group (0 vs. 9 [6.3%], P<0.001).
The descriptive statistics are summarized in Table 5. A total of 144 (30.5%) out of 457 polytrauma patients developed AKI. Out of these 144 patients, stage 1 AKI patients were 90 (62.5%), stage 2 were 28 (19.44%), stage 3 were 11 (7.6%), and stage 4 were 15 (10.41%). Thirteen patients (9.02%) required RRT. One hundred and twenty patients (83.33%) showed good renal recovery. Thirty-four patients (23.61%) died. None of the patients progressed to chronic renal failure.
Acute reduction of renal function is defined as AKI [14]. AKI after polytrauma is a common kidney-related complication; the reported incidence of AKI after polytrauma is 25%–40% [4-6]. In our study, the incidence of AKI after polytrauma is found to be 30.5%. Genetic predisposition to the development of end-stage renal disease has been observed [18], and there has been a recent growing interest to define genetics associated with AKI [18,19]. Because of the abundant expatriate population, Dubai gives a unique opportunity to study genetic predisposition in a specific disease. However in the present study, no differences are found in the incidence of AKI according to patients’ ethnicities (Table 1).
Trauma-induced AKI is more common in a patient with preexisting comorbidities [7]. This finding is confirmed in the present study; the CCI is higher is the AKI group. The usual source of polytrauma-induced AKI is either pre-renal or post-renal cause [8]. Also, the main causes of trauma-induced AKI found are shock, use of massive transfusion, rhabdomyolysis, and ACS. The shock here is hemorrhagic shock; and it is further evident from hyperlactatemia, low ScvO2, metabolic acidosis, and the higher need of massive transfusion in the AKI group. Other reported causes of trauma-induced AKI are contrast-induced nephropathy [20] and postoperative AKI [21]. Study population had received contrast media only once and had undergone major surgical procedure. The effect of these two factor is beyond comparison.
Another important finding is that the ISS is significantly higher in the AKI group compared to the Non-AKI group. There is good logistic regression between the severity of trauma (measured by ISS value) and the probability of developing AKI after trauma. ISS is an indispensable yet easy-to-calculate scoring system used in trauma patients. It is an anatomical score that measures the severity of polytraumas. It is calculated by summing an assigned abbreviated Injury Scale code and score from an internationally recognized dictionary that describes over 2,000 injuries and ranges from 1 (minor injury) to 6 (critical injury). There is a linear correlation between ISS and the severity of trauma [10]. High ISS is associated with hemorrhagic shock and coagulopathy thereby, increasing the requirement for blood products [22,23]. The eventuality of rhabdomyolysis and ACS after polytrauma is also correlated with high ISS [24-26]. Thus, in the present or earlier studies [4-8], whatever shock, the need for massive transfusion, rhabdomyolysis, and ACS observed are a mere reflection of severe polytrauma as evident from high ISS in the AKI group.
In the present study, patients who had developed trauma-induced AKI, the proportions of stages 1, 2, 3, and 4 of AKI were 62.5%, 19.44%, 7.6%, and 10.41% respectively. The requirement for RRT was observed in 9.02% of patients with AKI. Good renal recovery was observed in 83.33% of patients with AKI. These findings correlates with the metanalysis done by Søvik et al [8]. LOS-hospital, LOS-ICU, the need for mechanical ventilation, days spent on a ventilator, and mortality is significantly higher in the AKI group compared to the non-AKI group. This finding correlates with previous similar studies [4,27].
The merits of this study as compared to previously reported studies [4-6] are our sample size was large, we included severe polytrauma victims (ISS more than 25), we included subjects of diverse ethnicity, and we included subjects with polytrauma due to various causes (RTA, fall, or work-related injuries).
Implications of the study: from the result of this study it is evident that AKI is not uncommon in polytrauma victims and also it can affect the patient outcome after polytrauma. Following implications can be postulated form result of the study. (1) Setup and resources: a health care facility which is routinely managing polytrauma patient should have protocol for early recognition, prevention, and effective management of AKI in polytrauma victims. All the health care providers should keep themselves abreast about this vulnerable organ dysfunction. (2) Resuscitations: all the patient should receive optimal initial resuscitations with fluid or blood product as hemorrhagic shock is a main cause of AKI after trauma. On the other spectrum overzealous fluid resuscitations has its own hazardous like ACS, which is also one of the causes of AKI. So, it is imperative to have balance resuscitations effort which can be best achieved by forming institutional discrete goal directed therapy.
The present study has the following limitations. First, uniformity during initial resuscitation: our study is patient record-based, observational, and retrospective studies. We had not delineated the initial resuscitation protocol in polytrauma patients. Thus, it was difficult to determine whether all patients received uniform resuscitation, which is one of the determining factors for AKI after trauma. Second, outcome variables after AKI: patient’s LOS-hospital, LOS-ICU, need for mechanical ventilation, days spent on a ventilator, and mortality depend on the severity of trauma as indicated by high ISS score [23,28,29]. Similarly, AKI is also more common after severe polytrauma as indicated by the linear relationship between severe polytrauma and a high ISS score. Therefore, it is difficult to determine whether bad outcome variables are caused by AKI alone, or they are a reflection of severe polytrauma.
In conclusion, AKI is common after severe polytrauma. There is a statistically significant logistic regression between the severity of polytrauma and the occurrence of AKI. The incidence of AKI after polytrauma is about 30.5%. AKI is more common in a patient with preexisting comorbidities. The frequent causes of AKI after polytrauma are hemorrhagic shock, massive blood transfusion, rhabdomyolysis, and ACS. Stage 1 AKI is the most common type of AKI after polytrauma. Only a small proportion of patients will require RRT. The outcome of AKI after polytrauma is good, and none of the patients progressed to chronic renal failure. Although the LOS-hospital, LOS-ICU, the need for mechanical ventilation, days spent of mechanical ventilation, and mortality are significantly higher in the AKI group compared to the non-AKI group, this association may be related to more severe injuries in the AKI group. Finally, further prospective studies are needed to know the exact impact of AKI on outcome variables after polytrauma.
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: BSW, ZFA, AMAH. Data curation: BSW, GZA, GAKN, MSA, SS, MS. Formal analysis: BSW, ATYAK, HH. Funding acquisition: ZFA. Methodology: BSW, ZFA, AMAH. Project administration: BSW, ZFA. Visualization: ZFA, AMAH. Writing–original draft: BSW, GZA, SS, MS. Writing–review & editing: BSW, GZA, SS, MS.
REFERENCES
1. Elessawy F. The boom: population and urban growth of Dubai city. Horizons Hum Soc Sci. 2017; 2:26–41.

2. Bener A, Crundall D. Road traffic accidents in the United Arab Emirates compared to Western countries. Adv Transp Stud Int J. 2005; 6:5–12.
3. Barss P, Addley K, Grivna M, Stanculescu C, Abu-Zidan F. Occupational injury in the United Arab Emirates: epidemiology and prevention. Occup Med (Lond). 2009; 59:493–8.

4. Perkins ZB, Captur G, Bird R, Gleeson L, Singer B, O'Brien B. Trauma induced acute kidney injury. PLoS One. 2019; 14:e0211001.

5. Don Bosco D, Gangalal GM, Rao S, Chakrapani AT. Acute kidney injury in severe trauma patients: a record-based retrospective study. Adv J Emerg Med. 2019; 3:e22.
6. Muhamedhussein MS, Manji M, Nungu KS, Ruggajo P, Khalid K. Prevalence and risk factors of acute kidney injury in polytrauma patients at Muhimbili Orthopedic Institute, Tanzania. Afr J Emerg Med. 2021; 11:74–8.

7. Ahmed N, Mathew RO, Kuo YH, Md AA. Risk of severe acute kidney injury in multiple trauma patients: risk estimation based on a national trauma dataset. Injury. 2020; 51:45–50.

8. Søvik S, Isachsen MS, Nordhuus KM, Tveiten CK, Eken T, Sunde K, et al. Acute kidney injury in trauma patients admitted to the ICU: a systematic review and meta-analysis. Intensive Care Med. 2019; 45:407–19.

9. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011; 173:676–82.

10. Copes WS, Champion HR, Sacco WJ, Lawnick MM, Keast SL, Bain LW. The Injury Severity Score revisited. J Trauma. 1988; 28:69–77.

11. Patil V, Shetmahajan M. Massive transfusion and massive transfusion protocol. Indian J Anaesth. 2014; 58:590–5.

13. Martin D, Mantziari S, Demartines N, Hübner M, ESA Study Group. Defining major surgery: a Delphi consensus among European Surgical Association (ESA) members. World J Surg. 2020; 44:2211–9.

14. Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013; 17:204.

15. Sosa G, Gandham N, Landeras V, Calimag AP, Lerma E. Abdominal compartment syndrome. Dis Mon. 2019; 65:5–19.

16. Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017; 13:241–57.

17. Tandukar S, Palevsky PM. Continuous renal replacement therapy: who, when, why, and how. Chest. 2019; 155:626–38.
18. Ortega-Loubon C, Martínez-Paz P, García-Morán E, Tamayo-Velasco Á, López-Hernández FJ, Jorge-Monjas P, et al. Genetic susceptibility to acute kidney injury. J Clin Med. 2021; 10:3039.

19. Vilander LM, Kaunisto MA, Pettilä V. Genetic predisposition to acute kidney injury: a systematic review. BMC Nephrol. 2015; 16:197.
20. Li Y, Ren K. The mechanism of contrast-induced acute kidney injury and its association with diabetes mellitus. Contrast Media Mol Imaging. 2020; 2020:3295176.

21. Prowle JR, Forni LG, Bell M, Chew MS, Edwards M, Grams ME, et al. Postoperative acute kidney injury in adult non-cardiac surgery: joint consensus report of the Acute Disease Quality Initiative and PeriOperative Quality Initiative. Nat Rev Nephrol. 2021; 17:605–18.

22. Zhang L, Lin M, Tang X, Tang Y. Correlation between coagulation fibrinolysis function and outcomes during hospitalization in patients with severe traumatic hemorrhagic shock. Emerg Med Int. 2022; 2022:3775868.

23. Elgin LB, Appel SJ, Grisham D, Dunlap S. Comparisons of trauma outcomes and injury severity score. J Trauma Nurs. 2019; 26:199–207.

24. Assanangkornchai N, Akaraborworn O, Kongkamol C, Kaewsaengrueang K. Characteristics of creatine kinase elevation in trauma patients and predictors of acute kidney injury. J Acute Med. 2017; 7:54–60.
25. Holodinsky JK, Roberts DJ, Ball CG, Blaser AR, Starkopf J, Zygun DA, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care. 2013; 17:R249.

26. Tiwari AR, Pandya JS. Study of the occurrence of intra-abdominal hypertension and abdominal compartment syndrome in patients of blunt abdominal trauma and its correlation with the clinical outcome in the above patients. World J Emerg Surg. 2016; 11:9.

27. Skinner DL, Kong VY, de Vasconcellos K, Bruce JL, Bekker W, Laing GL, et al. Acute kidney injury on presentation to a major trauma service is associated with poor outcomes. J Surg Res. 2018; 232:376–82.

Figure 2.
Showing logistic regression model illustrating relation between acute kidney injury (AKI) and Injury Severity Score (ISS).
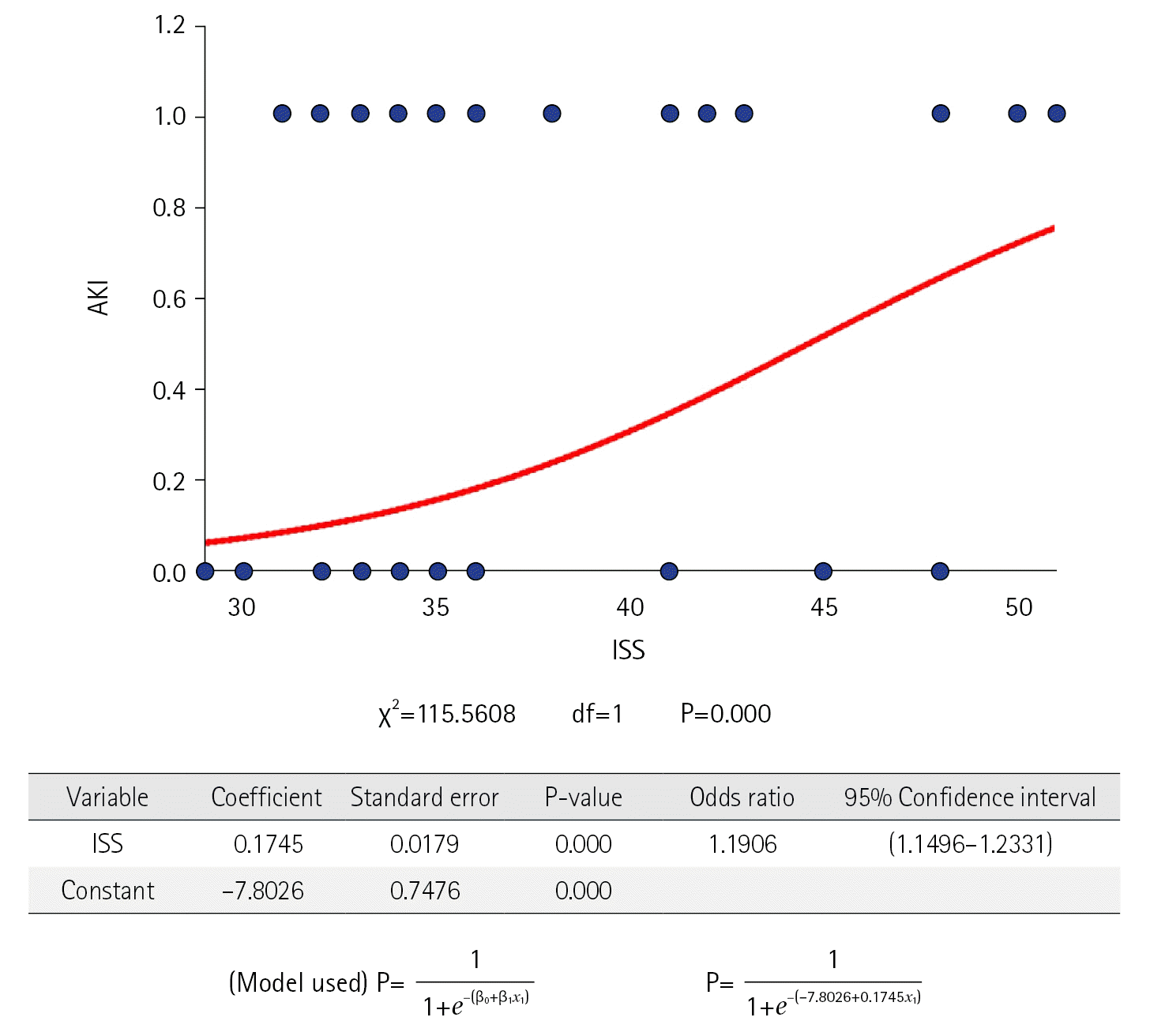
Table 1.
Demographics
| Variable | Non-AKI group (n=313) | AKI group (n=144) | P-value |
|---|---|---|---|
| Age (yr) | 0.349a) | ||
| 18–30 | 139 (44.4) | 61 (42.4) | |
| 31–40 | 93 (29.7) | 47 (32.6) | |
| 41–50 | 54 (17.3) | 18 (12.5) | |
| 51–60 | 27 (8.6) | 18 (12.5) | |
| Nationality | 0.975a) | ||
| Emirati | 39 (12.5) | 16 (11.1) | |
| Non-Emirati | |||
| Asian | 219 (70.0) | 100 (69.4) | |
| Middle east Asian | 29 (9.3) | 14 (9.7) | |
| African | 10 (3.2) | 6 (4.2) | |
| European+American | 16 (5.1) | 8 (5.6) | |
| Sex | 0.148a) | ||
| Male | 275 (87.9) | 133 (92.4) | |
| Female | 38 (12.1) | 11 (7.6) | |
| Mechanism of injury | 0.821a) | ||
| RTA | 212 (67.7) | 94 (65.3) | |
| Fall | 87 (27.8) | 42 (29.2) | |
| WR | 14 (4.5) | 8 (5.6) | |
| Body mass index (kg/m2) | 24.3 (22.0–27.0) | 25.3 (22.0–28.6) | 0.306b) |
| Charlson comorbidity index | 0.12±0.38 | 0.23±0.51 | 0.020c) |
| Injury Severity Score on admission | 36.0 (33.0–41.0) | 48.0 (37.5–48.0) | 0.001b) |
Table 2.
Cause of AKI
| Variable | Non-AKI group (n=313) | AKI group (n=144) | P-value |
|---|---|---|---|
| Shock in 24 hr | 0.001a) | ||
| Yes | 81 (25.9) | 133 (92.4) | |
| No | 232 (74.1) | 11 (7.6) | |
| On admission lactate | 2.3 (1.4–4.1) | 6.6 (4.5–8.9) | 0.001b) |
| On admission ScvO2 | 75 (75–75) | 59 (55–65) | 0.001b) |
| On admission base deficient (–) | 4 (2–6) | 10 (9–14) | 0.001b) |
| Massive transfusion | <0.001a) | ||
| Yes | 45 (14.4) | 131 (91.0) | |
| No | 268 (85.6) | 13 (9.0) | |
| Rhabdomyolysis | 0.001a) | ||
| Yes | 61 (19.5) | 57 (39.6) | |
| No | 252 (80.5) | 87 (60.4) | |
| Maximum value | 9,306 (73–12,304) | 9,768 (7,221–18,775) | 0.368b) |
| ACS | <0.001a) | ||
| Yes | 6 (1.9) | 23 (16.0) | |
| No | 307 (98.1) | 121 (84.0) |
Table 3.
Multivariate logistic regression
Table 4.
Outcome
| Variable | Non-AKI group (n=313) | AKI group (n=144) | P-value |
|---|---|---|---|
| LOS in hospital (day) | 28 (16–51) | 37 (18–74) | 0.006a) |
| LOS in ICU (day) | 12 (7–19) | 15 (9–23) | 0.003a) |
| Mechanical ventilation | <0.001b) | ||
| Yes | 255 (81.5) | 138 (95.8) | |
| No | 58 (8.9) | 6 (4.2) | |
| Ventilator day | 9 (5–16) | 7 (12–20) | 0.001a) |
| 28-Day mortality | 8 (2.6) | 25 (17.4) | 0.001b) |
Table 5.
Descriptive statistics of AKI group




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download