Abstract
Background
In patients with severe trauma, the diagnosis of acute kidney injury (AKI) is important because it is a predictive factor for poor prognosis and can affect patient care. The diagnosis and staging of AKI are based on change in serum creatinine (SCr) levels from baseline. However, baseline creatinine levels in patients with traumatic injuries are often unknown, making the diagnosis of AKI in trauma patients difficult. This study aimed to enhance the accuracy of AKI diagnosis in trauma patients by presenting an appropriate reference creatinine estimate (RCE).
Methods
We reviewed adult patients with severe trauma requiring intensive care unit admission between 2015 and 2019 (n=3,228) at a single regional trauma center in South Korea. AKI was diagnosed based on the current guideline published by the Kidney Disease: Improving Global Outcomes organization. AKI was determined using the following RCEs: estimated SCr75-modification of diet in renal disease (MDRD), trauma MDRD (TMDRD), admission creatinine level, and first-day creatinine nadir. We assessed inclusivity, prognostic ability, and incrementality using the different RCEs.
Results
The incidence of AKI varied from 15% to 46% according to the RCE used. The receiver operating characteristic curve of TMDRD used to predict mortality and the need for renal replacement therapy (RRT) had the highest value and was statistically significant (0.797, P<0.001; 0.890, P=0.002, respectively). In addition, the use of TMDRD resulted in a mortality prognostic ability and the need for RRT was incremental with AKI stage.
Acute kidney injury (AKI) is defined as a sudden decline in kidney function. AKI is an independent risk factor for increased mortality in patients with severe trauma admitted to the intensive care unit (ICU) [1-5]. In trauma patients, the incidence of AKI ranges widely from 1% to 50%, seemingly due to inconsistent diagnostic criteria and unclear estimates of reference creatinine estimates [6].
Accurate diagnosis and management of AKI can help improve survival in severely injured patients [7]. The diagnostic criteria for AKI have been developed over the past few decades. Recently, the Kidney Disease: Improving Global Outcomes (KDIGO) group proposed AKI diagnostic criteria based on urine output (UO) and serum creatinine (SCr) levels [8]. These criteria are used to diagnose and stage AKI according to the degree of increase in creatinine levels from baseline SCr or according to UO. However, baseline SCr is often unknown in trauma patients, making AKI diagnosis difficult.
There are no clear guidelines on how to estimate the reference creatinine in trauma patients; however, several methods have been proposed to estimate baseline SCr for AKI diagnosis in patients with severe trauma. This study aimed to determine an appropriate reference creatinine estimate for post-traumatic AKI diagnosis. We hypothesized that a more appropriate reference creatinine estimate is more strongly related to the diagnosis of AKI and its clinical outcomes, such as mortality. We assessed the ability of reference creatinine estimates to diagnose AKI based on incidence, prognosis, and incrementality by AKI stage and existing verified reports.
This study was reviewed and approved by the Institutional Review Board of Pusan National University Hospital (No. H-2004-020-090). Owing to its retrospective design, informed consent was waived.
In this retrospective cohort study, we reviewed adult patients with severe trauma from the Korea Trauma Database (KTDB) between January 1, 2015, and December 31, 2019 at Pusan National University Hospital, a high-volume regional trauma center in Busan, South Korea. A total of 4,293 patients had severe trauma (Injury Severity Score [ISS] ≥16). The exclusion criteria were age <16 years, ICU admission time <12 hours, known chronic kidney disease, and unclear laboratory results or medical records. Accordingly, 3,228 patients were enrolled in the study (Figure 1). Data on demographic characteristics, initial vital signs, medical history, injury type, length of ICU admission, and mortality were obtained from the KTDB. In addition, laboratory results for seven days were extracted from the medical records.
AKI was defined and staged based on the current KDIGO guidelines (Table 1) [8]. Initiation of renal replacement therapy (RRT) corresponds to stage 3 AKI in the KDIGO guidelines [8]. However, this study did not include RRT as a diagnostic criterion. RRT was included as an outcome variable rather than a diagnostic criterion in our study. In addition, the KDIGO 2012 guidelines include the UO criterion; however, this study did not apply it because the accuracy of the available data could not be verified.
We identified some reference creatinine estimates that were used when the patient's baseline creatinine level was unknown in the trauma literature. The modification of diet in renal disease (MDRD) equation used for back-calculation was developed for estimating the glomerular filtration rate (GFR) and is widely accepted [9]. The equation is as follows:
The estimated serum creatinine 75 (eSCr75)-MDRD back-calculates the MDRD equation to estimate unidentified SCr, assuming a lower normal GFR (75 ml/min/1.73 m2), according to international recommendations [8]. The trauma MDRD (TMDRD) was also designed to estimate creatinine in the young and generally healthy trauma population; however, it uses the highest median GFR (121 ml/min/1.73 m2) demonstrated by trauma patients during the first week of admission [10]. The admission creatinine level was defined as the first SCr measurement after arrival at the hospital [11], and the first-day nadir was defined as the lowest SCr measured within 24 hours of arrival [12].
This study assessed four reference creatinine estimates (eSCr75-MDRD, TMDRD, admission creatinine, and first-day nadir) with the following primary outcomes: inclusivity, prognostic ability, and incrementality. Inclusivity was assessed based on AKI incidence. Prognostic ability was assessed using the estimated odds ratio (OR) of mortality, initiation of RRT, and area under the curve (AUC). Incrementality was assessed by evaluating whether mortality and RRT also increased as the AKI stage increased.
Continuous variables were presented as median and interquartile ranges (IQRs), and categorical variables were presented as numbers and percentages. The categorical variables were compared using the chi-square test when appropriate; otherwise, Fisher’s exact test was used. Continuous variables were compared using the Wilcoxon rank-sum test based on the distribution. Modified Poisson regression analysis was performed to estimate the OR. This method has been proposed as an alternative to log binomial models when convergence is a problem (as was the case in this study). We used the receiver operating characteristic (ROC) curve and AUC to evaluate the prognostic factors predicting mortality. Statistical significance was defined as P≤0.05. All statistical analyses were performed using IBM SPSS ver. 20.0. (IBM Corp.) and Stata ver. 14.2 (StataCorp.).
In total, 3,228 patients were included in the analysis. The proportion of men was higher (77%), and the median age was 57 years (IQR, 44–68 years). Most of the damage mechanisms were blunt injuries (97%), and the median ISS was 25 (IQR, 19–29). The reference creatinine estimate ranged widely from 0.67 mg/dl (when TMDRD was used) to 1.00 mg/dl (when eSCr75-MDRD was used). The participant characteristics are presented in Table 2.
In univariate analysis, patients with AKI diagnosed using any of the reference creatinine estimates were more severely injured, older, and had a larger arrival base deficit, higher mortality rate, and higher rate of RRT requirement compared with those without AKI. This trend was the same regardless of the reference creatinine estimate used. Patients with AKI diagnosed using admission creatinine levels had no statistically significant difference in arrival systolic blood pressure (SBP). The incidence of AKI varied among the reference creatinine estimates, from 15% (when the admission creatinine level was used) to 46% (when the TMDRD was used) (Table 3).
The estimated OR of increased mortality and RRT showed associations with AKI diagnosed using all reference creatinine estimates after adjusting for age, arrival SBP, ISS, and arrival base deficit (Table 4). This result is similar to the significant increase in mortality and RRT according to AKI diagnosis previously identified in the univariate analysis (P<0.001). A ROC curve analysis according to each reference creatinine estimate was performed to determine the sensitivity, specificity, and positive and negative predictive values of mortality and RRT by AKI stage. The results are presented in Table 5. The AUCs of TMDRD in predicting the mortality and initiation of RRT were higher than those of the other estimates, and this difference was statistically significant (AUC: 0.797, P<0.001; AUC: 0.890, P=0.002) (Figure 2).
The estimated OR of mortality and RRT by AKI stage showed that it increased with each increase in AKI stage, and all were statistically significant after adjusting for age, arrival SBP, ISS, and arrival base deficit (Table 6). The mortality rate and requirement of RRT according to AKI stage was calculated for each reference creatinine estimate (Figure 3). The association of the increase in mortality and RRT with the increasing stage only occurred with TMDRD, and not in the other reference creatinine estimates.
In this study, there were variations in the strength of association between AKI diagnosis and mortality based on the reference creatinine estimate used. The TMDRD reference creatinine estimate was the most relevant in terms of incidence, prognosis, and incrementality, and it was the most appropriate for AKI diagnosis according to our hypothesis. AKI in patients with severe trauma admitted to the ICU is a common complication with considerable mortality. Moreover, severe injury is a strong risk factor for AKI [1-5]. Indeed, trauma patients experience single or multiple exposures to AKI risk factors, including severe trauma, hypovolemic shock, rhabdomyolysis, and abdominal compartment syndrome [2,13-16].
According to several guidelines, a diagnosis of AKI should be made based on an increase in SCr from the reference value. However, the choice of reference creatinine estimate for post-traumatic AKI diagnosis remains controversial. Various approaches have been used to define reference creatinine estimates [17,18]. Common approaches include the use of admission creatinine, the lowest inpatient creatinine, or other surrogates, such as MDRD. Without reliable baseline SCr, the KDIGO 2012 guideline recommends that SCr be estimated using the back-calculated MDRD equation assuming a lower normal GFR of 75 mL/min/1.73 m2 [8]. Although a recent study questioned the reliability of estimated creatinine clearance, several studies have reported that eSCr75-MDRD appears applicable in critical care [17,19,20]. In contrast, TMDRD may be more appropriate than eSCr75-MDRD for the diagnosis and prognosis of AKI in the trauma population [10,18]. In our study, TMDRD appeared to be more useful than other estimates (eSCr75-MDRD, admission creatinine, and first-day nadir) as a reference creatinine estimate for the diagnosis of AKI in patients with severe trauma.
The incidence of AKI in trauma patients is reportedly in the range of 1%–76% because of the different AKI criteria, reference creatinine estimates, levels of trauma severity, and length of follow-up used [6,21-23]. In our study, there was a wide range in AKI incidence, from 15% (when admission creatinine was used) to 46% (when TMDRD was used). This result is comparable to that previously reported. In a recent systematic review and meta-analysis of trauma patients admitted to critical care, the overall incidence of AKI was 20.4% without a clear reference creatinine estimate. The incidence of AKI increased by 31.9% in studies that focused mainly on blunt injuries [21]. In another systematic review and meta-analysis of trauma patients in the ICU, the overall mean incidence of post-traumatic AKI was 24% (95% confidence interval [CI], 20%–29%), with considerable heterogeneity in significant outcomes [22].
According to a large cohort study, the in-hospital mortality of patients with AKI was 27% after adjusting for the KDIGO stage and differences in age, sex, and severity of illness (OR, 1.13–2.20; P<0.001) in various patients admitted to the ICU [24]. A meta-analysis of trauma patients found that the mortality rate in patients with AKI was 27% (95% CI, 20%–35%) [22]. Haines et al. [21] reported that the pooled relative risk of death from AKI was 3.6 (95% CI, 2.4–5.3). In our study, the estimated OR of mortality ranged from 4.3 (when the first-day creatinine nadir was used) to 9.0 (when admission creatinine was used). The estimated OR of mortality of TMDRD was 4.6 (95% CI, 3.5–6.1). In particular, the AUC value of TMDRD was higher than that of the other estimates, and this difference was statistically significant (Figure 2A). The TMDRD reference creatinine estimate resulted in a diagnosis that was prognostic of both mortality and incrementality for each AKI stage (Figure 3A). Although there were some differences in patient characteristics and diagnostic criteria between our study and the others, the mortality rate of TMDRD (25.7%) was comparable to that in the other observations [21,22,24].
RRT is the only supportive measure in patients with severe AKI. The KDIGO guidelines recommend immediate initiation of RRT if an absolute indication exists [8]. The initiation of RRT before the onset of major complications has conceivable advantages for patients with severe AKI [25]. In our study, the AUC value of TMDRD used to predict the requirement of RRT was higher than that of the other estimates, and this difference was statistically significant (Figure 2B). The association of the increase in RRT with the increasing stage only occurred with TMDRD and not in the other reference creatinine estimates (Figure 3B).
There are three main differences between our study and other studies that have suggested methods of approximating reference creatinine estimates to diagnose AKI in patients with trauma. First, our study was based on a relatively large number of patients and compared the adequacy of previously known reference creatinine estimates in patients with severe trauma. Saour et al. [10] assessed MDRD performance in predicting SCr in a severe trauma population of 775 patients. In contrast, 3,228 patients were included in our study. Second, the severity of trauma in patients included in the present study was more than that in patients included in the other studies. Saour et al. [10] reported a mean ISS of 19. Hatton et al. [23] reported a median ISS of 20. In contrast, the median ISS in this study was 25. We believe that the difference in results between our study and others may have been influenced by the severity of trauma. Third, in contrast to the diversity in population composition and trauma mechanisms in other studies, all patients in our study were Asian (100%), and 97% had suffered blunt injuries. In the study by Hatton et al. [23], most participants were Caucasian (52%) or Hispanic (22%), while African Americans comprised only 17% of the population and Asians only 2%. In addition, participants with a blunt injury accounted for 85% of the cohort [23]. Those authors reported that eSCr75-MDRD may be more useful than TMDRD for the diagnosis and prognosis of AKI [23]. In contrast, TMDRD appeared to be a more appropriate reference creatinine estimate than eSCr75-MDRD in our study. This is thought to be on account of the differences in race composition and injury mechanism. Although the role of race adjustment in estimating kidney function is controversial [26,27], we believe that racial differences must be considered when selecting an appropriate reference value for creatinine.
This study had several limitations. First, UO could not be applied as a diagnostic criterion because of our inability to accurately verify UO. UO records before admission to the ICU (via the emergency, operating, or angiography room) were missing. In studies where both SCr and UO were used as diagnostic criteria, the incidence of AKI increased from 24% based on SCr alone to 52% when UO was added as a diagnostic criterion. Moreover, the risk of death was greatest when patients met both SCr levels and UO criteria for AKI [28,29]. Thus, our study may have underestimated the incidence and mortality of AKI. Second, mortality or initiation of RRT is one of the strongest indicators of prognosis in patients with AKI. However, there are several other indicators of prognosis, such as complications, duration of RRT, and renal disease progression to chronic kidney disease. However, investigation of this aspect was limited by model structure. Finally, this was a single-center retrospective study, and the results were insufficient for drawing conclusions. Additional multicenter, prospective, randomized controlled trials are necessary to confirm the validity of TMDRD as a reference creatinine estimate.
In the current study, TMDRD was the most appropriate reference creatinine estimate (in terms of inclusivity, prognostic ability, and incrementality) for diagnosing and staging post-traumatic AKI. When using TMDRD as a diagnostic criterion, the incidence of AKI in patients with severe trauma was approximately 46%. If complementary prospective randomized controlled, multicenter comparative studies are conducted in the future, we believe a more definitive conclusion can be reached.
▪ Acute kidney injury (AKI) in patients with severe trauma is an important prognostic factor; however, diagnosing AKI is challenging because the baseline creatinine level for a definitive diagnosis is not known.
▪ Currently, there is no clear consensus on the estimated baseline creatinine level that is diagnostic of AKI in patients with severe trauma.
▪ In this study, we compared the diagnostic ability of several reference creatinine estimates (RCEs) and found trauma modification of diet in renal disease to be the most suitable RCE for AKI diagnosis.
Notes
REFERENCES
1. Bihorac A, Delano MJ, Schold JD, Lopez MC, Nathens AB, Maier RV, et al. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg. 2010; 252:158–65.
2. Eriksson M, Brattström O, Mårtensson J, Larsson E, Oldner A. Acute kidney injury following severe trauma: Risk factors and long-term outcome. J Trauma Acute Care Surg. 2015; 79:407–12.
3. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005; 294:813–8.
4. Lai WH, Rau CS, Wu SC, Chen YC, Kuo PJ, Hsu SY, et al. Post-traumatic acute kidney injury: a cross-sectional study of trauma patients. Scand J Trauma Resusc Emerg Med. 2016; 24:136.
5. Harrois A, Soyer B, Gauss T, Hamada S, Raux M, Duranteau J, et al. Prevalence and risk factors for acute kidney injury among trauma patients: a multicenter cohort study. Crit Care. 2018; 22:344.
6. Harrois A, Libert N, Duranteau J. Acute kidney injury in trauma patients. Curr Opin Crit Care. 2017; 23:447–56.
7. National Institute for Health and Care Excellence. Acute kidney injury: prevention, detection and management of acute kidney injury up to the point of renal replacement therapy [Internet]. London: National Institute for Health and Care Excellence;2019. [cited 2022 Jul 28]. Available from: https://www.nice.org.uk/guidance/ng148.
8. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012; 120:c179–84.
9. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med. 2006; 354:2473–83.
10. Saour M, Klouche K, Deras P, Damou A, Capdevila X, Charbit J. Assessment of modification of diet in renal disease equation to predict reference serum creatinine value in severe trauma patients: lessons from an observational study of 775 cases. Ann Surg. 2016; 263:814–20.
11. Brandt MM, Falvo AJ, Rubinfeld IS, Blyden D, Durrani NK, Horst HM. Renal dysfunction in trauma: even a little costs a lot. J Trauma. 2007; 62:1362–4.
12. Podoll AS, Kozar R, Holcomb JB, Finkel KW. Incidence and outcome of early acute kidney injury in critically-ill trauma patients. PLoS One. 2013; 8:e77376.
13. Hultström M. Neurohormonal interactions on the renal oxygen delivery and consumption in haemorrhagic shock-induced acute kidney injury. Acta Physiol (Oxf). 2013; 209:11–25.
14. Elterman J, Zonies D, Stewart I, Fang R, Schreiber M. Rhabdomyolysis and acute kidney injury in the injured war fighter. J Trauma Acute Care Surg. 2015; 79(4 Suppl 2):S171–4.
15. Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009; 361:62–72.
16. Mohmand H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol. 2011; 22:615–21.
17. Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006; 10:R73.
18. Bagshaw SM, George C, Gibney RT, Bellomo R. A multi-center evaluation of early acute kidney injury in critically ill trauma patients. Ren Fail. 2008; 30:581–9.
19. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure: definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8:R204–12.
20. Kadivarian S, Heydarpour F, Karimpour H, Shahbazi F. Measured versus estimated creatinine clearance in critically ill patients with acute kidney injury: an observational study. Acute Crit Care. 2022; 37:185–92.
21. Haines RW, Fowler AJ, Kirwan CJ, Prowle JR. The incidence and associations of acute kidney injury in trauma patients admitted to critical care: a systematic review and meta-analysis. J Trauma Acute Care Surg. 2019; 86:141–7.
22. Søvik S, Isachsen MS, Nordhuus KM, Tveiten CK, Eken T, Sunde K, et al. Acute kidney injury in trauma patients admitted to the ICU: a systematic review and meta-analysis. Intensive Care Med. 2019; 45:407–19.
23. Hatton GE, Du RE, Pedroza C, Wei S, Harvin JA, Finkel KW, et al. Choice of reference creatinine for post-traumatic acute kidney injury diagnosis. J Am Coll Surg. 2019; 229:580–8.
24. Srisawat N, Sileanu FE, Murugan R, Bellomod R, Calzavacca P, Cartin-Ceba R, et al. Variation in risk and mortality of acute kidney injury in critically ill patients: a multicenter study. Am J Nephrol. 2015; 41:81–8.
25. STARRT-AKI Investigators; Canadian Critical Care Trials Group; Australian and New Zealand Intensive Care Society Clinical Trials Group; United Kingdom Critical Care Research Group; Canadian Nephrology Trials Network; Irish Critical Care Trials Group, et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020; 383:240–51.
26. Lee K, Kim H, Ryu D. Choice of reference value of creatinine for diagnosing post-traumatic acute kidney injury: consider ethnic and racial disparities. J Am Coll Surg. 2020; 231:497.
27. Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA. 2019; 322:113–4.
28. Macedo E, Malhotra R, Bouchard J, Wynn SK, Mehta RL. Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int. 2011; 80:760–7.
29. Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015; 26:2231–8.
Figure 1.
Flowchart of the study. ISS: Injury Severity Score; ICU: intensive care unit; CKD: chronic kidney disease.
Figure 2.
Comparison of estimated serum creatinine 75 (eSCr75) modification of diet in renal disease (MDRD), trauma MDRD (TMDRD), admission creatinine, and first-day nadir creatinine area under the curve (AUC) for predicting mortality (A) and the need for renal replacement therapy (B). Values are presented as AUC (95% confidence interval).
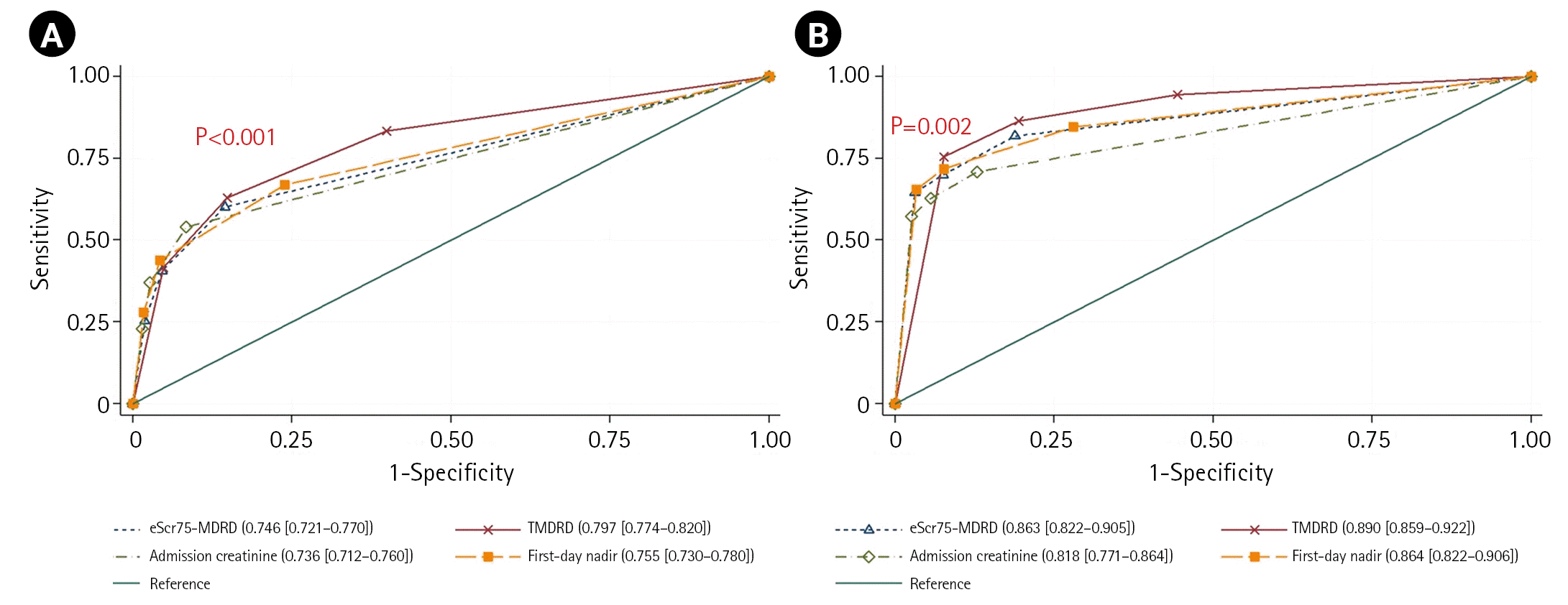
Figure 3.
Mortality (A) and the need for renal replacement therapy (B) by acute kidney injury (AKI) stage. eSCr75: estimated serum creatinine 75; MDRD: modification of diet in renal disease.
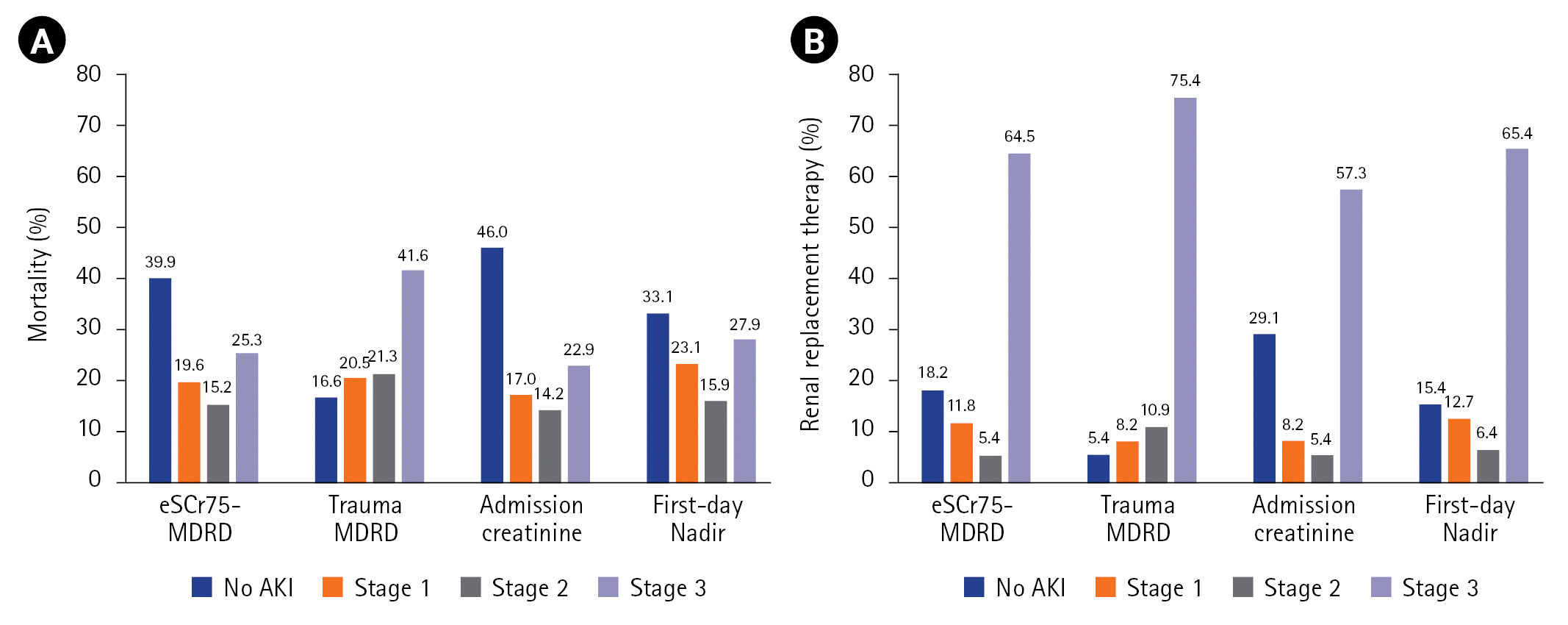
Table 1.
KDIGO acute kidney injury definition and stage
Table 2.
Characteristics of study participants
Table 3.
Characteristics of patients with and without acute kidney injury, by reference creatinine estimate
Table 4.
Estimated odds ratio of mortality and renal replacement therapy by acute kidney injury diagnosis, adjusted for age, arrival systolic blood pressure, Injury Severity Score, and arrival base deficit
Table 5.
Sensitivity, specificity, PPV, and NPV value of mortality and renal replacement therapy by acute kidney injury stage
Table 6.
Estimated odds ratio of mortality and renal replacement therapy by acute kidney injury stage, adjusted for age, arrival systolic blood pressure, Injury Severity Score, and arrival base deficit




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download