Abstract
The collection of air in the cranial cavity is called pneumocephalus. Although simple pneumocephalus is a benign condition, accompanying increased intracranial pressure can produce a life-threatening condition comparable to tension pneumothorax, which is termed tension pneumocephalus. We report a case of tension pneumocephalus after drainage of a cerebrospinal fluid hygroma. The tension pneumocephalus was treated with decompression craniotomy, but the patient later died due to the complications related to critical care. Traumatic brain injury and neurosurgical intervention are the most common causes of pneumocephalus. Pneumocephalus and tension pneumocephalus are neurosurgical emergencies, and anesthetics and intensive care management like the use of nitrous oxide during anesthesia and positive pressure ventilation have important implications in their development and progress. Clinically, patients can present with various nonspecific neurological manifestations that are indistinguishable from those of a primary neurological condition. If the diagnosis is questionable, patients should be investigated using computed tomography of the brain. Immediate neurosurgical consultation with decompression is the treatment of choice.
The collection of air in the cranial cavity is called pneumocephalus. Simple pneumocephalus is a benign condition that occurs commonly after head trauma or postoperatively after neurosurgery [1]. Tension pneumocephalus (TPC) refers to increased intracranial pressure (ICP) and herniation of brain structures. It is a rare life-threatening condition comparable to tension pneumothorax [1].
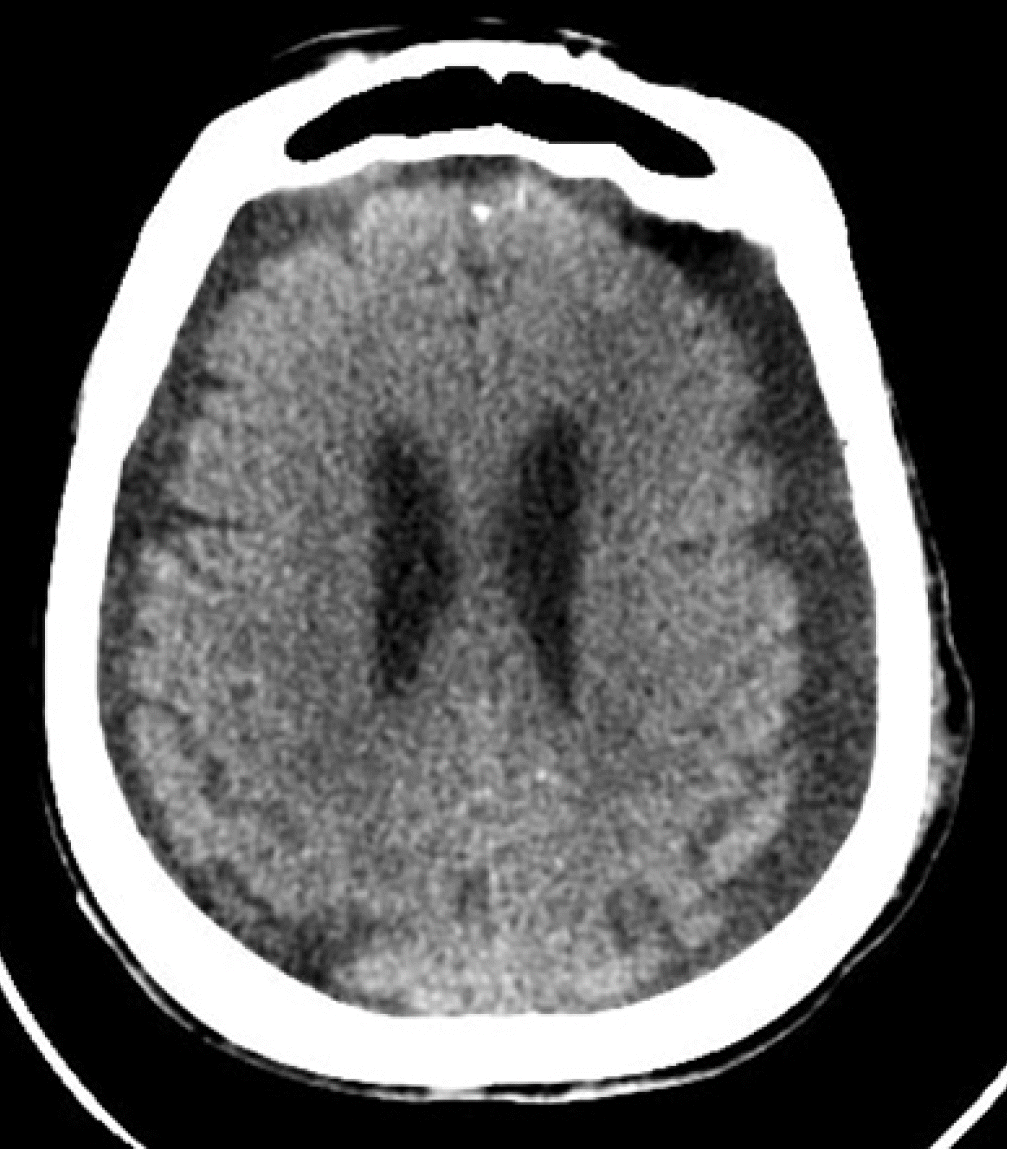
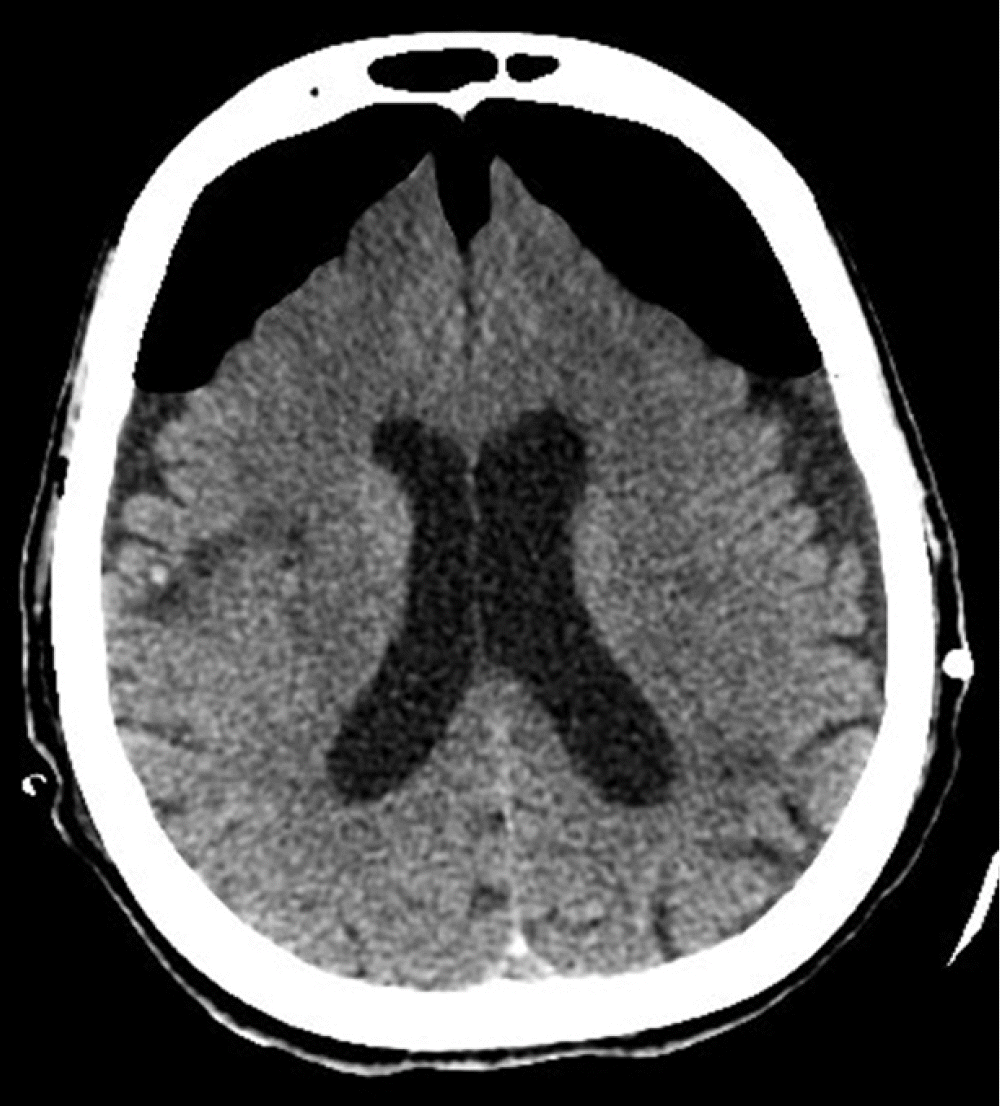
Our patient was an 84-year-old female with a past medical history of diabetes mellitus, hypertension, paroxysmal atrial fibrillation, and an old cerebrovascular event (left middle cerebral artery infarction) with no residual weakness. She was ambulatory with acceptable cardiorespiratory status for her age. Following a fall, she was admitted to our hospital with a left intertrochanteric femur fracture. On the next day, an orthopedic surgeon performed Gama nailing of the femur. The postoperative course was uneventful. On the 7th postoperative day; however, she developed right-sided hemiparesis and altered mental status. The Glasgow coma scale (GCS) was 10/15 (E4, V1, M5). Noncontrast computed tomography (NCCT) of the brain suggested bilateral cerebrospinal fluid (CSF) hygroma more strongly affecting the left side than the right side at the cerebral convexity (Figure 1). She was referred to a neurosurgeon and subsequently underwent a left parietal Burr hole and evacuation of CSF hygroma under general anesthesia. She was extubated in the postoperative period and remained in an intensive care unit (ICU) for neuro observation. Postoperatively, her GCS was 15/15 (E4, V5, M6), but she had persistent right-sided hemiparesis, which was similar to her preoperative state. Six hours postoperatively, she developed generalized tonic-clonic seizures, and her GCS dropped to 5/15 (E1, V1, M3). She underwent immediate intubation to secure her airway. She was sedated with a continuous infusion of fentanyl and midazolam, and she was connected to mechanical ventilation. She also received antiepileptic levetiracetam. Another NCCT of the brain revealed near-complete evacuation of the bilateral CSF hygroma and development of the classic “Mount Fuji” sign, indicating the formation of TPC (Figure 2). Her management included maintenance in a flat position in bed, 100% oxygen, sedation, an antiepileptic, and other brain-protective measures. She underwent immediately surgery, and a neurosurgeon performed left frontoparietal decompressive craniectomy under general anesthesia. Postoperatively, she was kept in the ICU under sedation. On the second postoperative day, a repeat NCCT of the brain showed near-complete resolution of the pneumocephalus. Her sedation was stopped, but her GCS did not improve beyond 9/15 (E4, V1, M4). On the 7th postoperative day, she underwent a tracheotomy for low GCS. Unfortunately, the patient developed other complications associated with her critical illness: ventilator-associated pneumonia, sepsis, acute kidney injury, and multi-organ failure. Finally, she succumbed to death on the 25th postoperative day. Because the patient was deceased, we obtained consent from her son for publication of this case report. This case report was approved by the head of the ICU of our hospital.
Pneumocephalus or pneumatocele is defined as the presence of gas within any intracranial compartment of the cranial vault: intraventricular, intraparenchymal, subarachnoid, subdural, or epidural. The term “pneumocephalus” was described first by Wolff in 1914 [2]. In 1962, Kessler and Strern [3] reported a case of pneumocephalus that caused brain herniation leading to rapid deterioration and death. They described the term “tension pneumocephalus,” which is the same as tension pneumothorax and requires immediate intervention.
The most common cause of pneumocephalus is traumatic head injury. The reported incidence of pneumocephalus after a head injury is 0.5% to 1% [4]. Other common causes of pneumocephalus include (1) craniofacial trauma especially involving the skull base [5]; (2) intra- or extracranial neurosurgical an intervention like drainage of subdural hemorrhage, shunt surgeries, or CSF drainage [5]; (3) spinal or epidural injections [5]; (4) otorhinolaryngological procedures [5]; (5) infections involving the brain or middle ear such as brain abscess, encephalitis, meningitis, and otitis [5]; (6) brain tumors [5]; (7) positive pressure ventilation, either invasive or non-invasive [6]; (8) nitrous oxide as an anesthetic during anesthesia [7]; (9) air travel (a small pneumocephalus can grow due to pressure changes at high altitude) [5]; (10) barotrauma, hyperbaric oxygen therapy [5]; and (11) radiotherapy to the cranium [5].
Several mechanisms have been postulated in the pathophysiology of pneumocephalus. (1) The inverted soda bottle effect occurs when CSF is lost due to any cause. This loss will decrease the ICP, and air will be sucked into the cranial cavity to replace the lost volume of CSF [5]. (2) The ball valve mechanism occurs when the extra-cranial pressure is greater than the ICP (e.g., sneezing, coughing, and straining), and air enters the cranial cavity. The affected intracranial structure seals the entry site, and the flow of air is sparse [5]. This mechanism is responsible for pneumocephalus associated with positive pressure ventilation [5,6]. (3) During epidural injections, pneumocephalus mainly develops if a loss of resistance technique is used to identify the epidural space [5]. The air can enter the meninges due to accidental injection, inadvertent dural puncture, or pressure differences between the intraventricular/cisternal space and atmosphere [5]. (4) During anesthesia, pneumocephalus can develop due to the use of nitrous oxide during anesthesia. The blood–gas partition coefficient of nitrous oxide is 34 times greater than that of nitrogen, allowing nitrous oxide to diffuse into the cranial vault faster than nitrogen or air [7]. (5) During air travel, a small pneumocephalus can increase in size and can lead to TPC. Boyle's law states that if the temperature remains fixed in a confined space like the cranial cavity, the absolute pressure and volume are inversely proportional. Thus, when absolute pressure decreases (e.g., during a flight), the volume of air increases proportionally [5].
The clinical features of pneumocephalus and TPC are similar and nonspecific. The most common symptoms include nausea, vomiting, headache, altered mental status, and convulsions [5,8]. Neurological findings include nuchal rigidity, photophobia, and focal neurological deficit [5,8]. In cases of TPC, clinical deterioration is due to increased ICP and the Cushing reflex [8]. The patient can present with bradycardia, hypertension, or even cardiac arrest [8]. If the patient has an ICP monitoring device, it can show a high reading [8]. When there is doubt between simple pneumocephalus and TPC, the patient should be investigated, as simple pneumocephalus does not usually require decompressive surgery [8].
Pneumocephalus can be investigated by plain X-ray of the skull and computed tomography (CT) of the brain [5]. Although X-ray of the skull can detect a large pneumocephalus, it is seldom used with the advancement of CT; CT of the brain is the investigation of choice [9]. When pneumocephalus is detected on brain CT, the patient should be investigated for a skull base fracture or CSF leak [1]. Radiological signs described for diagnosis of pneumocephalus/TPC are the peaking sign, Mount Fuji sign, and air bubble sign [5,9]. The peaking sign is bilateral compression of the frontal lobes caused by trapped air in the subdural space. This sign demonstrates no separation of the frontal lobes and is more common in pneumocephalus than in TPC [5,9]. The Mount Fuji sign is bilateral compression and separation of the frontal lobes due to trapped subdural and interhemispheric space air. It is observed as non-attenuating collections formed between the two frontal lobes and is specific to TPC [5,9]. The air bubble sign is the presence of multiple air bubbles scattered around the cisterns. It is caused mainly by a tear in the arachnoid membrane and is more common in TPC [5,9].
A simple pneumocephalus, even if it is massive, can be managed conservatively [10]. Conservative management involves the supine position, 100% supplemental oxygen, and frequent neurological monitoring to detect any signs/symptoms of increased ICP [7]. However, if the patient demonstrates any features of high ICP that indicate the development of TPC, an early neurosurgical referral, neurosurgical decompression, and repair of the causative defect are recommended [8]. Without timely intervention, TPC ultimately can lead to increased ICP, brain stem herniation, coma, and death [5].
In our case, the patient developed pneumocephalus by the “inverted soda bottle effect” after drainage of CSF, and she clinically presented with acute seizures and a decrease in GCS, which indicated increased ICP. Thus, it was concluded that she did not have simple postoperative pneumocephalus but TPC. The reported incidence of pneumocephalus after the evacuation of CSF or blood from subdural space is 2.5% [8]. In our case, TPC was confirmed by a brain CT that showed the “Mount Fuji” sign, which is specific to TPC [5,9]. We managed this patient by keeping her in the supine position to prevent further CSF leaks, and she was given 100% supplemental oxygen to hasten the removal of intracranial nitrogen (air). Some authors, like Heidari [10] have reported successful conservative management over neurosurgical decompression in a patient with a massive pneumocephalus. Options of neurosurgical decompression include needle decompression either blind or under radiological guidance through an existing burr hole or craniotomy [1], controlled decompression via a closed water-seal drainage system [11], ventriculostomy for air in the ventricle [8], emergency decompression by creating new cranial Burr holes [8], and decompressive craniectomy [8]. Individual performance, outcome, or superiority of one procedure over those of another are not well studied in the reported medical literature, but there is a theoretical risk of intracranial infection with needle/drain decompression [12]. The choice of neurosurgical decompression technique depends on surgical expertise, institutional protocol, and availability of resources. In our patient, we managed TPC with left frontoparietal decompressive craniectomy, which resulted in seizure control and improvement of GCS.
Pneumocephalus can be prevented by using the following measures. (1) When using noninvasive respiratory support (high-flow nasal cannula or continuous positive pressure mask) in a patient with a history of head trauma, keep the flow rate or pressure as low as possible and closely monitor for any neurologic worsening or development of new neurological symptoms [6]. (2) When using invasive ventilation, avoid high airway pressure and hyperventilation [6]. (3) During anesthesia, avoid the use of nitrous oxide as an anesthetic agent in neurosurgical patients [7]. (4) During epidural injections, use saline instead of air to identify the epidural space [5]. (5) Most physicians recommend avoiding air travel for 2–8 weeks after surgery [13].
Although pneumocephalus and TPC are neurosurgical emergencies, their anesthetic and intensive care management have some important implications during development and progress. Intensivists must maintain an in-depth knowledge of the etiopathogenesis of these conditions so that they can be diagnosed promptly, and the patient can be referred to a neurosurgeon for immediate surgical decompression. Thus, proper ICU and anesthetic management can prevent the development of pneumocephalus and its progress.
REFERENCES
1. Harvey JJ, Harvey SC, Belli A. Tension pneumocephalus: the neurosurgical emergency equivalent of tension pneumothorax. BJR Case Rep. 2016; 2:20150127.
2. Wolff E. Luftansammlung im rechten seitenventrikel des gehirns (pneumozephalus). Münch Med Wochenschr. 1914; 61:899.
3. Kessler LA, Stern WZ. The ventriculopleural shunt procedure for hydrocephalus: case report of an unusual complication. J Pediatr. 1962; 60:418–20.
4. Lee SH, Koh JS, Bang JS, Kim MC. Extensive tension pneumocephalus caused by spinal tapping in a patient with Basal skull fracture and pneumothorax. J Korean Neurosurg Soc. 2009; 45:318–21.

5. Sweni S, Senthilkumaran S, Balamurugan N, Thirumalaikolundusubramanian P. Tension pneumocephalus: a case report with review of literature. Emerg Radiol. 2013; 20:573–8.

6. Chang Y, Kim TG, Chung SY. High-flow nasal cannula-induced tension pneumocephalus. Indian J Crit Care Med. 2020; 24:592–5.

10. Heidari SF. A patient with massive pneumocephalus without sign of tension pneumocephalus. J Intensive Crit Care. 2016; 2:3.

11. Arbit E, Shah J, Bedford R, Carlon G. Tension pneumocephalus: treatment with controlled decompression via a closed water-seal drainage system: case report. J Neurosurg. 1991; 74:139–42.
12. Das JM, Bajaj J. Pneumocephalus [Internet]. StatPearls Publishing;2021. [cited 2021 Sep 11]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535412.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download