In our case, the patient experienced delayed recovery of consciousness, language, and motor response due to catatonia for several weeks following total elbow arthroplasty under general anesthesia. The persistence of postoperative unconsciousness is of particular concern to anesthesiologists. We suggest that catatonia may be a potential cause of delayed emergence after general anesthesia.
Case Report
Written informed consent was obtained from the patient and her family for the publication of this case report. The Institutional Review Board (IRB) waived the review of this case report (IRB No. 202212007). This case report was written according to the recommendations of the CAse REport (CARE) guideline [
3].
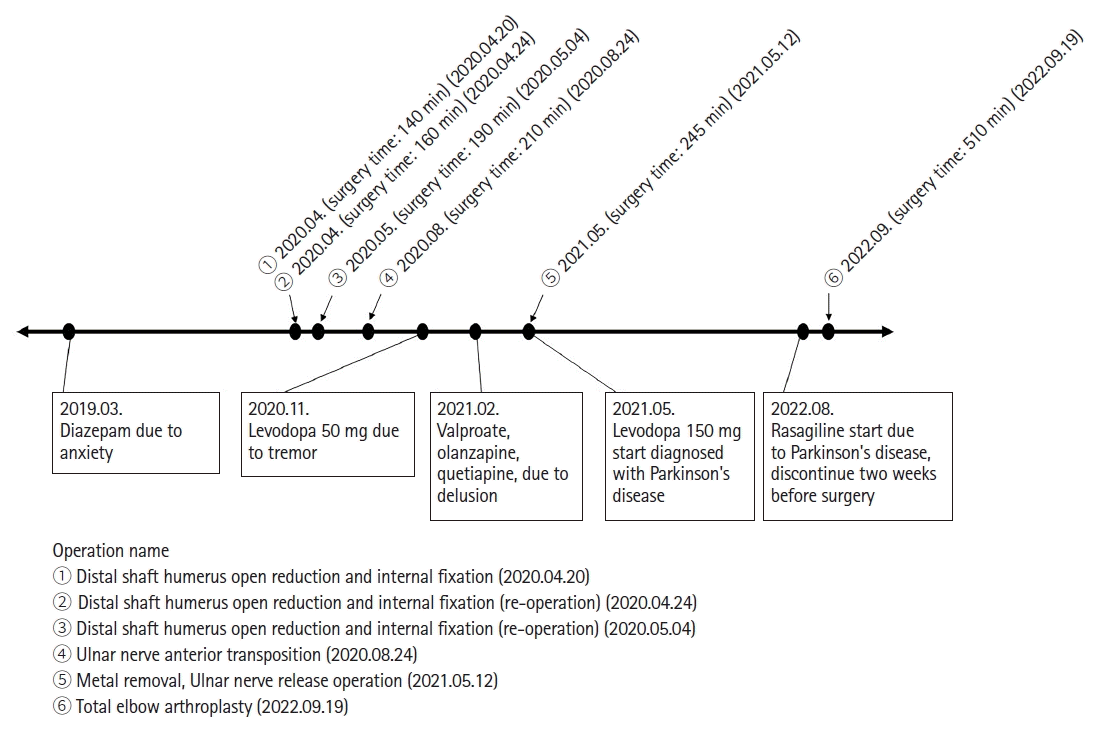
A 68-year-old woman (height: 162 cm, weight: 52 kg) underwent scheduled total elbow arthroplasty under general anesthesia. Her medical history included well-controlled diabetes, Parkinson’s disease, depression, anxiety, and dementia. She was taking valproate, olanzapine, quetiapine, donepezil, diazepam, levodopa, rasagiline, and pregabalin. Of these, only rasagiline was discontinued two weeks before surgery. Two years ago, she was admitted to a closed psychiatric ward after having experienced delusions and intermittent drowsiness for one year. Furthermore, she had undergone orthopedic surgery under general anesthesia five times in the last two years due to a distal humerus shaft fracture. No perioperative events occurred during the previous surgeries (
Fig. 1).
Before anesthesia induction, the patient could articulate her name and date of birth without showing any tremors associated with Parkinson’s disease. Her initial blood pressure was 145/86 mmHg, and her heart rate was 78 beats/min. For induction, 1 mg/kg of propofol, 0.2 µg/kg/min remifentanil, and 0.6 mg/kg of rocuronium were used. Anesthesia was maintained using desflurane, remifentanil, and intermittent rocuronium while targeting a mean arterial pressure of 70 mmHg with invasive monitoring, a bispectral index (BIS) of 40–60, and a train-of-four (TOF) ratio of less than 30% throughout the surgery. We administered 70 mg of rocuronium from the start of anesthesia to the end of the surgery. The total anesthesia time was 510 min, and 3,100 ml of crystalloid was administered. Total urine output was 540 ml. During the surgery, the patient’s mean arterial pressure was maintained at > 70 mmHg by administering 5 mg of ephedrine bolus or 100 µg phenylephrine bolus with continuous phenylephrine infusion at 0.4 µg/kg/min.
At the end of the operation, all anesthetics were stopped, and muscle relaxation was reversed with 200 mg of sugammadex. The patient had a TOF of 90% or higher and was able to breathe spontaneously, but her BIS repeatedly fell to 50–60 after increasing to 90 or higher. When we opened her eyelids, her eyes were tightly closed and deviated left or downward. The patient remained semi-comatose, and her neck and right arm were stiff. Despite the administration of pain stimuli, the patient did not respond. At that time, the patient’s body temperature was 37.1°C, and arterial blood gas analysis was within the normal range.
The patient’s mental status did not change over one hour after the operation; therefore, we consulted a neurologist and decided to perform computed tomographic angiography (CTA) for stroke evaluation while the patient remained intubated. However, CTA images did not reveal any obvious stroke. The patient was transferred to an intensive care unit. Three hours after the operation, the patient’s consciousness gradually improved from a semi-coma to a stupor. A neurologist suspected that prolonged anesthesia had caused toxic encephalopathy.
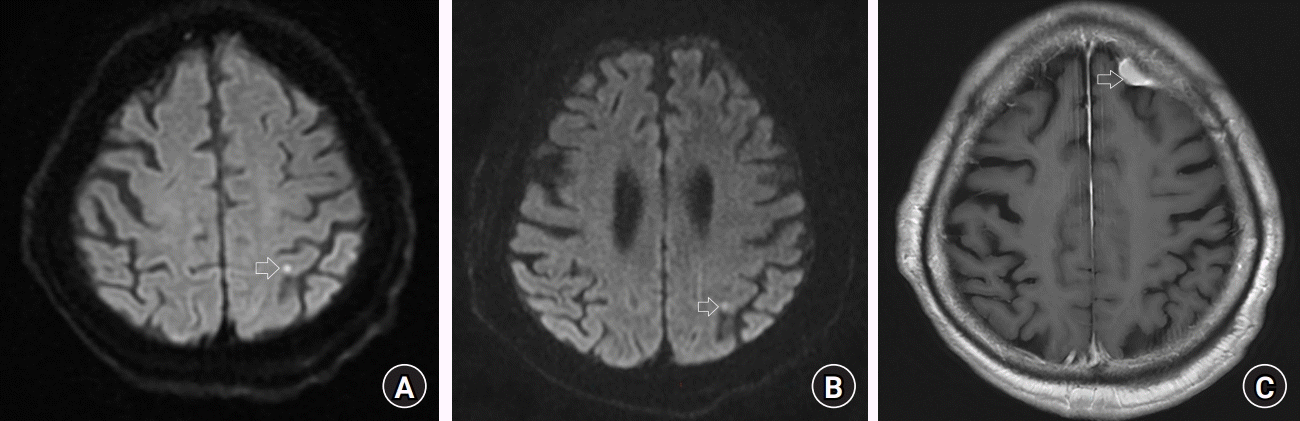
On postoperative day (POD) 1, an electroencephalogram (EEG) revealed a focal seizure pattern from the left posterior temporal area and diffuse cerebral dysfunction. A diffusion magnetic resonance imaging (MRI) scan performed on the same day showed a high signal on the diffusion-weighted image in the left parietal cortex, indicating subacute infarction (
Fig. 2A). However, the neurologist believed that the patient’s decreased level of consciousness was not due to subacute infarction. Despite this, the patient remained stuporous and continued to exhibit left-sided and downward eyeball deviations. To rule out postoperative epileptic seizures, we started the patient on an anti-epileptic drug (levetiracetam) and readministered Parkinson’s disease medications on POD 2. On that day, the patient could follow simple commands, had stable vital signs, maintained self-respiration, and was extubated. However, on POD 3, she could only open her eyes when called, responded minimally to simple commands, and had a right-leg tremor. Her Glasgow Coma Scale score was 10 (E3, V3, and M4).
Although brain MRI and EEG revealed no suspected lesions, the patient exhibited persistent aphasia, immobility, and unresponsiveness. On POD 6, follow-up MRI and EEG scans showed that the lesion in the left parietal cortex remained unchanged (
Fig. 2B) compared to the previous diffusion-weighted image taken on POD 1. However, an extra-axial enhancing mass with T2 weighted image high/T1 weighted image iso signal intensity, as well as diffusion restriction, suggesting meningioma with a diameter of 1.3 cm, was seen along the left frontal convexity (
Fig. 2C). Although this patient had a meningioma, it was unlikely that this condition would result in neurological symptoms. A neurologist performed a follow-up EEG under the suspicion of hypoactive delirium and epileptic seizure. No epileptiform discharges were observed on her EEG on POD 6, but her unresponsiveness persisted. Finally, the patient was diagnosed with psychosomatic catatonia and was transferred to the psychiatric department. The patient was transferred to a general ward on POD 11. Her symptoms improved after receiving a lorazepam injection, and she showed normal EEG findings on POD 22 (
Table 1).
On POD 23, the patient was mentally alert but continued to exhibit little spontaneous speech or activity. As the patient had slightly improved with lorazepam, the psychiatrist planned electroconvulsive therapy (ECT) on POD 60. After undergoing five rounds of ECT, the patient could make eye contact, walk with a walker, and respond to simple commands. The patient’s rigidity and cognitive function improved, and she was discharged from the hospital on POD 93 with outpatient follow-up.
Discussion
Our patient, who was taking medications for mental illness and Parkinson’s disease, experienced delayed awakening and was in a semi-comatose state after prolonged surgery. Several examinations were initially performed to exclude organic causes of mental status changes; however, no evident lesions were found. The patient’s condition subsequently improved after receiving treatment for catatonia in the neurological and psychiatric departments.
Unresponsiveness during recovery from general anesthesia can have various etiologies. After excluding anesthesia-related complications and cardiovascular and electrolyte abnormalities, psychogenic causes should be considered [
4]. Prolonged postoperative coma is relatively rare, with estimates of 0.005%–0.08% after general surgery [
5]. Postoperative coma is independently correlated with old age, urgent surgery, pre-existing brain injury, perioperative hypotension, postoperative organ failure, and infection. In this patient, CTA and MRI showed a left parietal lobe microinfarct and left frontal meningioma that was unlikely to cause neurological symptoms. EEG showed a focal seizure wave from the left posterior temporal area and diffuse cerebral dysfunction, suggesting an epileptic seizure. After administering anti-epileptic medications, the follow-up EEG on POD 23 was normal, but the patient’s decreased mental status persisted. However, laboratory tests and imaging did not reveal any abnormalities.
In the initial postoperative period, the patient presented with a stuporous mental state that led us to consider hypoactive delirium as a possible cause. Hypoactive delirium is common, accounting for 50% of all postoperative delirium [
6]. It typically manifests within minutes to hours after the patient regains consciousness, and the EEG shows a slowing pattern. However, our patient did not regain consciousness after surgery and showed a seizure pattern on the EEG, leading us to exclude hypoactive delirium as a possibility.
Importantly, the patient was taking levodopa and rasagiline for Parkinson’s disease. Rasagiline was discontinued two weeks before surgery, so neuroleptic malignant syndrome (NMS) can be considered a differential diagnosis. NMS is a severe disorder caused by an adverse reaction to medications with dopamine receptor antagonist properties or the rapid withdrawal of dopaminergic drugs. The symptoms of NMS include fever, muscle rigidity, and altered mental status [
7]. However, NMS seems unlikely since our patient discontinued rasagiline two weeks before surgery and continued to take levodopa until the surgery. Additionally, our patient did not present with a fever, so we were able to rule it out.
Our patient’s responses during the recovery phase of anesthesia, such as left-sided and downward eyeball deviations, neck and arm stiffness, and loss of pain response, are presumed to be manifestations of nonconvulsive status epilepticus (NCSE). NCSE is a type of status epilepticus characterized by the absence of prominent motor activity, instead presenting with symptoms such as stupor, staring, and unresponsiveness. NCSE can develop in patients with various clinical diagnoses, including hypoxic-anoxic encephalopathy, cancer, autoimmune disorders, drug toxicity, pregnancy, infections, alcohol intoxication or withdrawal, and central nervous system lesions. NCSE shows symptoms similar to catatonia; therefore, the differential diagnosis should be made cautiously using EEG clues [
8]. Early diagnosis and treatment are important because NCSE can result in permanent neurological sequelae. Continuous EEG recordings play a crucial role in patient prognosis. Although the patient had a seizure pattern on EEG from the end of anesthesia until POD 22, she remained unresponsive even after the EEG became normal, and the psychiatrist diagnosed her with stuporous catatonia.
Catatonia is classically associated with mental illness but can also occur in medical and neurological disorders. In the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), the current diagnostic criteria for catatonia require the presence of three or more of the following symptoms: stupor, waxy flexibility, catalepsy, mutism, posturing, negativism, stereotypes, mannerisms, grimacing, agitation, echopraxia, and echolalia. The pathophysiology of catatonia is not fully understood but is thought to be related to aberrant neuronal activity in different motor pathways and defective neurotransmitter regulation. One hypothesis suggests that dysregulation of γ-aminobutyric acid (GABA)-A, glutamate, and dopamine neurotransmitter systems may be a possible cause [
2]. Catatonia has also been associated with many medications, including benzodiazepine-derived medicines, and can occur due to the toxic effect of drugs or withdrawal from them [
9]. It is speculated that prolonged use of these medications increases GABA activity and that discontinuation may increase excitatory neurotransmission, leading to catatonia [
2]. In addition, drugs used in general anesthesia, including inhalation anesthetics, act on GABA receptors [
10]. It is still unclear whether general anesthesia directly triggers the occurrence of catatonia. Therefore, in our patient’s case, it is likely that multiple factors, such as using anesthetic drugs for a prolonged period, surgical stimulation-induced neuroinflammation, and psychiatric history, act in a complex manner to induce catatonia. Additionally, more than 75% of catatonia cases are related to abnormalities in brain imaging, and diffuse white matter lesions have been observed in many cases [
11]. In our case, the patient showed subacute infarction in the parietal cortex on diffusion MRI.
Catatonia is initially treated with lorazepam. If this is ineffective, ECT is considered a secondary treatment. ECT is the definitive treatment for catatonia that involves providing patients with short electrical brain stimulation under anesthesia. It has been proven effective in treating catatonia, especially when initiated early. If ECT is not available, glutamate antagonists and anti-epileptics can be used. In addition, atypical antipsychotic agents can be administered along with lorazepam [
12]. Our patient showed a slight improvement when treated with lorazepam, but after ECT, the patient could make eye contact, engage in short communication, and ambulate using a walker.
The reported cases of postoperative catatonia vary depending on the type of surgery and patient history, and it is potentially more common after liver transplantation than in other general surgery cases [
2]. Chacko et al. [
13] reported a suspected case of catatonia in a 64-year-old woman who had emerged without incident but lost consciousness and became unresponsive after a few minutes. Under suspicion of catatonia, midazolam 3 mg was administered, and she regained consciousness after 10 min.
Risk factors for catatonia include bipolar disorder, autism, schizophrenia, major depressive disorder, and mixed psychiatric disorders. Our patient had Parkinson's disease and depression and had been taking many medications, including diazepam, for three years. These factors are thought to have contributed to the development of catatonia, as the drug was discontinued the day before surgery [
2]. In addition, she appeared to have NCSE on awakening from anesthesia due to long-term surgery, and the catatonia persisted afterwards. It is difficult to exclude the possibility of a combination of NCSE and catatonia, as the EEG showed seizure waves until POD 22.
In conclusion, anesthesiologists should know the patient’s medication and history before surgery. Although uncommon, in patients who have been taking psychiatric drugs for a long time, catatonia should be considered a differential diagnosis if there is no response and delayed awakening after general anesthesia without any organic causes.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download