1. Stein ML, Park RS, Kovatsis PG. Emerging trends, techniques, and equipment for airway management in pediatric patients. Paediatr Anaesth. 2020; 30:269–79.
2. Hunyady A, Polaner D. Pediatric airway management education and training. Paediatr Anaesth. 2020; 30:362–70.
3. Black AE, Flynn PE, Smith HL, Thomas ML, Wilkinson KA; Association of Pediatric Anaesthetists of Great Britain and Ireland. Development of a guideline for the management of the unanticipated difficult airway in pediatric practice. Paediatr Anaesth. 2015; 25:346–62.
4. Lee JH, Park S, Jang YE, Kim EH, Kim HS, Kim JT. The distance between the glottis and the cuff of a tracheal tube placed through three supraglottic airway devices in children: a randomised controlled trial. Eur J Anaesthesiol. 2019; 36:721–7.
5. Kovatsis PG. Continuous ventilation during flexible fiberscopic-assisted intubation via supraglottic airways. Paediatr Anaesth. 2016; 26:457–8.
6. Burjek NE, Nishisaki A, Fiadjoe JE, Adams HD, Peeples KN, Raman VT, et al. Videolaryngoscopy versus fiber-optic intubation through a supraglottic airway in children with a difficult airway: an analysis from the multicenter pediatric difficult intubation registry. Anesthesiology. 2017; 127:432–40.
7. Wohl H. The cusum plot: its utility in the analysis of clinical data. N Engl J Med. 1977; 296:1044–5.
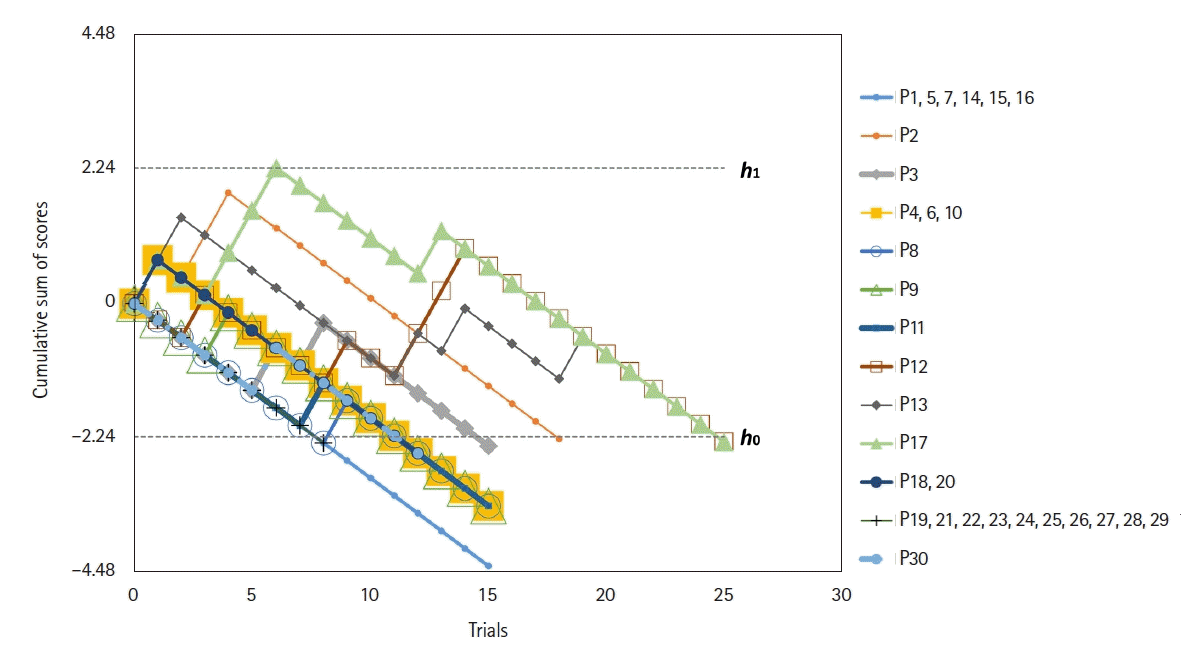
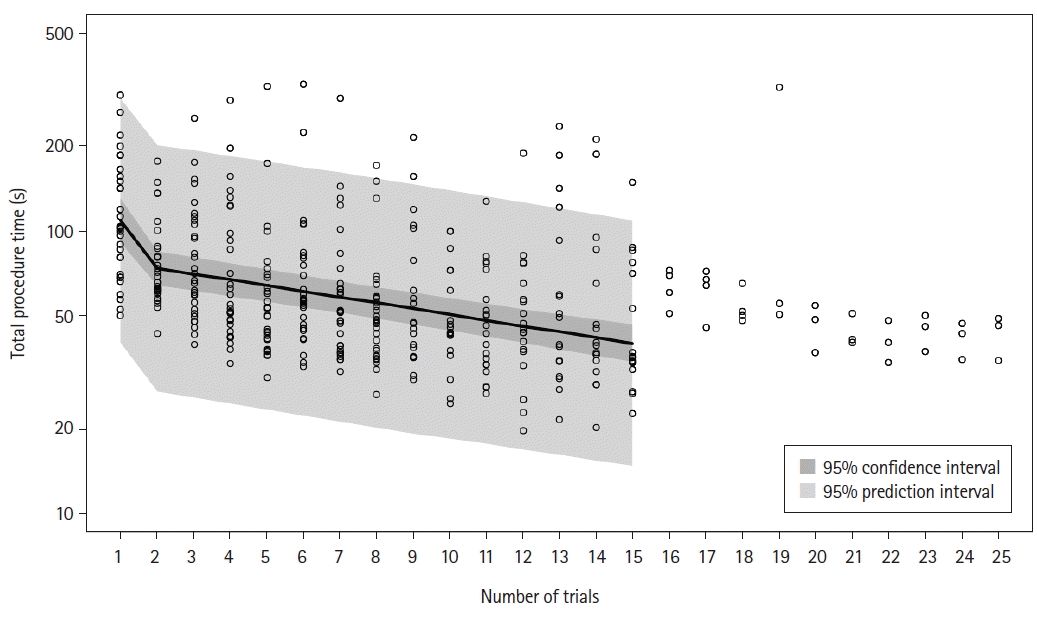
8. de Oliveira Filho GR, Helayel PE, da Conceicao DB, Garzel IS, Pavei P, Ceccon MS. Learning curves and mathematical models for interventional ultrasound basic skills. Anesth Analg. 2008; 106:568–73.
9. Orhan-Sungur M, Altun D, Özkan-Seyhan T, Aygün E, Koltka K, Çamcı E. Learning curve of ultrasound measurement of subglottic diameter for endotracheal tube selection in pediatric patients. Paediatr Anaesth. 2019; 29:1194–200.
10. Altun D, Ozkan-Seyhan T, Camci E, Sivrikoz N, Orhan-Sungur M. Learning curves for two fiberscopes in simulated difficult airway scenario with cumulative sum method. Simul Healthc. 2019; 14:163–8.
11. Ogrinc G, Armstrong GE, Dolansky MA, Singh MK, Davies L. SQUIRE-EDU (Standards for QUality Improvement Reporting Excellence in Education): publication guidelines for educational improvement. Acad Med. 2019; 94:1461–70.
12. Moser B, Keller C, Audige L, Dave MH, Bruppacher HR. Fiberoptic intubation of severely obese patients through supraglottic airway: a prospective, randomized trial of the Ambu® AuraGain™ laryngeal mask vs the i-gel™ airway. Acta Anaesthesiol Scand. 2019; 63:187–94.
13. Gasteiger L, Oswald E, Keplinger M, Putzer G, Luger M, Neururer S, et al. A randomized trial comparing the Ambu(R) Aura-i and the Ambu(R) Aura Gain laryngeal mask as conduit for tracheal intubation in children. Minerva Anestesiol. 2020; 86:1143–50.
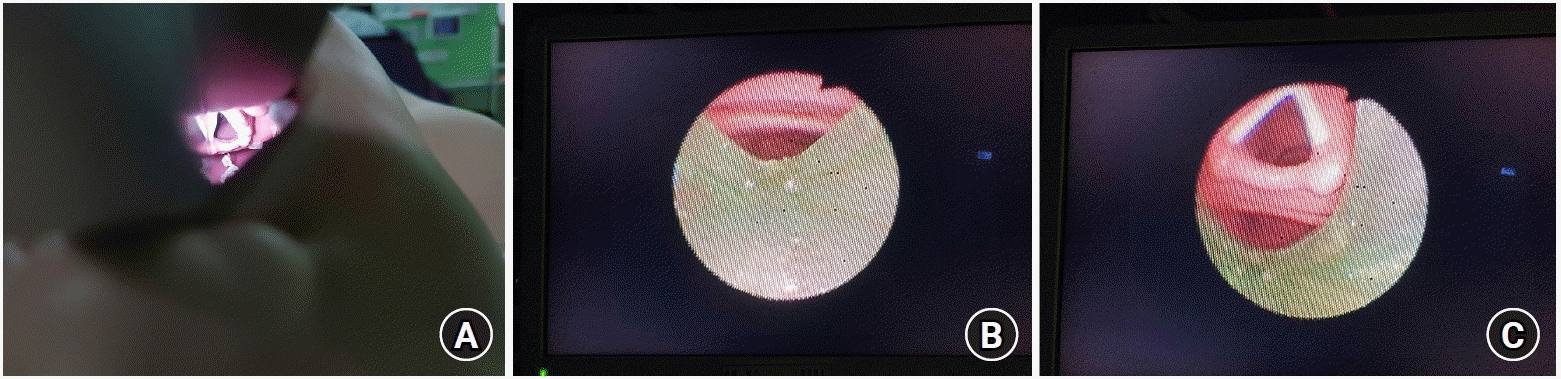
14. Brimacombe J, Berry A. A proposed fiber-optic scoring system to standardize the assessment of laryngeal mask airway position. Anesth Analg. 1993; 76:457.
15. Dalal PG, Dalal GB, Pott L, Bezinover D, Prozesky J, Bosseau Murray W. Learning curves of novice anesthesiology residents performing simulated fibreoptic upper airway endoscopy. Can J Anaesth. 2011; 58:802–9.
16. Kleine-Brueggeney M, Theiler L, Urwyler N, Vogt A, Greif R. Randomized trial comparing the i-gel™ and Magill tracheal tube with the single-use ILMA™ and ILMA™ tracheal tube for fibreoptic-guided intubation in anaesthetized patients with a predicted difficult airway. Br J Anaesth. 2011; 107:251–7.
17. Che Omar S, Hardy Mohamad Zaini R, Fui Wong T, Nazaruddin W, Hassan WM. Comparison of the Air-Q intubating laryngeal mask airway and the Ambu AuraGain laryngeal mask airway as a conduit for fibreoptic assisted endotracheal intubation for simulated cervical spine injury. Anaesthesiol Intensive Ther. 2021; 53:241–5.
18. Kim HJ, Park HS, Kim SY, Ro YJ, Yang HS, Koh WU. A randomized controlled trial comparing Ambu AuraGain and i-gel in young pediatric patients. J Clin Med. 2019; 8:1235.
19. Lee JH, Nam S, Jang YE, Kim EH, Kim HS, Kim JT. Clinical performance of Ambu AuraGainTM versus i-gelTM in anesthetized children: a prospective, randomized controlled trial. Anesth Pain Med (Seoul). 2020; 15:173–80.
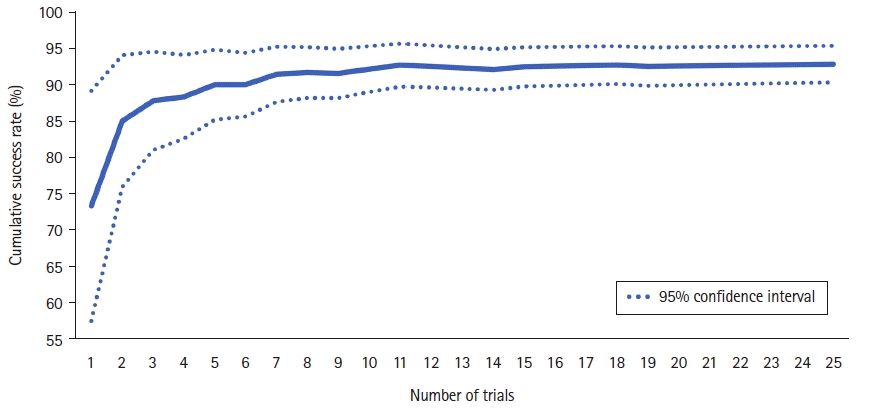
20. Kestin IG. A statistical approach to measuring the competence of anaesthetic trainees at practical procedures. Br J Anaesth. 1995; 75:805–9.
21. Jagannathan N, Roth AG, Sohn LE, Pak TY, Amin S, Suresh S. The new air-Q intubating laryngeal airway for tracheal intubation in children with anticipated difficult airway: a case series. Paediatr Anaesth. 2009; 19:618–22.
22. Asai T, Shingu K. Difficulty in advancing a tracheal tube over a fibreoptic bronchoscope: incidence, causes and solutions. Br J Anaesth. 2004; 92:870–81.
23. Kristensen MS. The Parker Flex-Tip tube versus a standard tube for fiberoptic orotracheal intubation: a randomized double-blind study. Anesthesiology. 2003; 98:354–8.
24. Brull SJ, Wiklund R, Ferris C, Connelly NR, Ehrenwerth J, Silverman DG. Facilitation of fiberoptic orotracheal intubation with a flexible tracheal tube. Anesth Analg. 1994; 78:746–8.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download