Abstract
Background
The shivering effect after spinal anesthesia in total knee arthroplasty (TKA) is challenging for anesthesiologists. This study aimed to compare two administration routes of dexmedetomidine as a post-neuraxial shivering prevention measure and an adjunctive analgesic and sedative agent.
Methods
Fifty-six patients were randomly allocated into two equal groups. The intravenous dexmedetomidine (IV dex) group received an IV infusion of 0.5 µg/kg dexmedetomidine diluted in 20 ml saline and an adductor canal block (ACB) consisting of 20 ml of 0.25% levobupivacaine and 1 ml saline. The adductor canal block dexmedetomidine (ACB dex) group received a 20 ml IV infusion of saline and an ACB consisting of 20 ml 0.25% levobupivacaine and 1 ml of 0.5 µg/kg dexmedetomidine.
Results
The incidence of shivering 1 h post spinal anesthesia was equal in both groups (50%); however, the shivering grade was significantly lower in the IV dex group 1 h postoperatively. The onset of sensory block was significantly later in the IV dex group (22.14 ± 2.52 min) than in the ACB dex group (12 ± 3.31 min). Postoperative analgesic duration (h) was significantly longer in the ACB dex group (12.28 ± 4.47) compared to the IV dex group (9.28 ± 1.90). The sedation scores were also significantly higher in the IV dex group in the preoperative, intraoperative, and immediate postoperative periods.
Total knee arthroplasty (TKA) is associated with severe post-operative pain. Early mobilization after TKA leads to better functional results and reduces the related complications; therefore, pain control with early motor function is the main goal for TKA [1,2].
The adductor canal block (ACB) is a nerve block of Hunters canal, which includes the saphenous and vastus medialis nerves. Additionally, other sensory nerves, such as the femoral nerve, medial cutaneous nerve, and both the anterior branch and terminal end of the posterior branch of the obturator nerve to some extent as it enters the distal part of the canal are blocked by the ACB [3]. The ACB is primarily considered a pure sensory nerve block because the only motor nerve it affects is the nerve to the vastus medialis muscle. Therefore, ACB provides adequate analgesia during knee surgery without affecting quadriceps strength [4,5].
Spinal anesthesia is often associated with an impaired thermoregulation system through the constraint of tonic vasoconstriction with subsequent temperature regulation. Moreover, spinal anesthesia redistributes the core heat from the trunk (below the block level) to the periphery. Consequently, patients are predisposed to hypothermia and shivering after spinal anesthesia [6]. Shivering can induce complications, particularly in patients with low cardiac and pulmonary reserves, through an increase in cardiac and systemic energy expenditure (metabolic rate is increased up to 400%), oxygen consumption, carbon dioxide production, and lactic acidosis [7].
Definitive prevention and treatment of shivering are necessary to decrease related complications and increase post-anesthesia comfort [8]. Dexmedetomidine is a highly selective Alpha-2 adrenergic agonist that is effective and safe as an analgesic adjuvant via various routes, including the intravenous (IV) [9], neuraxial [10], perineural [11], intramuscular, intranasal, and buccal routes [12,13]. However, few previous studies have focused on the shivering prevention effect of dexmedetomidine. Additionally, no studies have investigated the best route of dexmedetomidine administration to either prevent the occurrence of shivering after spinal anesthesia or decrease its severity.
This study thus aimed to investigate the optimal administration route of dexmedetomidine as 1) a preventive agent against neuraxial shivering and 2) an adjunctive analgesic and sedative agent. The incidence of shivering 1 h post spinal anesthesia was the primary outcome. The secondary outcomes included hemodynamics, postoperative pain scores, analgesic duration, total analgesic consumption on the first postoperative day, and sedation scores.
This prospective, randomized, comparative, double-blind study was conducted after approval from the Mansoura Faculty of Medicine Institutional Research Board (code R.18.11.342) and registration on ClinicalTrials.gov (NCT04266145). Written informed consent was received from all 56 adult patients with American Society of Anesthesiologists physical status classifications of I and II scheduled for unilateral primary TKA under spinal anesthesia. All procedures were conducted in accordance with the 2013 Declaration of Helsinki.
The exclusion criteria were as follows: severe hepatic and neuromuscular disease; chronic opioid use; known allergy to the study drugs; or any contraindications to regional anesthesia, such as patient refusal, coagulopathy, or injection site infections.
Preoperatively, patients were carefully assessed and instructed on the 100 mm visual analog scale (VAS) for pain assessment (0 mm = no pain; 100 mm = worst possible pain). Baseline preoperative VAS scores at rest were recorded. Patients fasted for 6 h for solids and 2 h for clear fluids.
Heart rate (HR), non-invasive mean arterial pressure (MAP), and peripheral oxygen saturation (SpO2) monitoring were initiated upon arrival at the pre-anesthetic room. Patients were randomly allocated into two groups (28 patients in each group) according to the route of dexmedetomidine administration using opaque coded envelopes. In the first group (IV dex), 0.5 µg/kg of dexmedetomidine diluted in 20 ml of normal saline was prepared for IV administration and the ACB was conducted with 20 ml of 0.25% levobupivacaine and 1 ml normal saline. In the second group (ACB dex), a 20 ml IV infusion of normal saline was administered and the ACB was conducted with 20 ml of 0.25% levobupivacaine and 1 ml of 0.5 µg/kg of dexmedetomidine.
Anesthetic drugs for both IV infusion and the ACB were prepared according to the group by an anesthesiologist who was not involved in the injection or perioperative assessment. Levobupivacaine hydrochloride 0.5% (Chirocaine; CuuridaAS, Abbvie, Italy) and preservative-free dexmedetomidine hydrochloride (Precedex, Hospira Inc., Canada) were used. The IV prepared solution was administered directly over 30 min, followed by a continuous infusion of warmed Ringer’s acetate at 7 ml/kg/h.
The ACB was performed immediately after administering the IV solution. A high-frequency linear ultrasound transducer was used, with the thigh abducted and externally rotated. The transducer was placed transverse to the longitudinal axis of the thigh, approximately halfway between the inguinal crease and superior margin of the patella. The deep femoral artery was identified between the vastus medialis and adductor longus muscles deep in the sartorius muscle. The saphenous nerve just lateral to the artery was identified if possible. Two milliliters of lidocaine 2% was injected lateral to the transducer, and an 18-gauge Tuohy needle was inserted in-plane from the lateral side of the transducer in a lateral-to-medial orientation through the sartorius muscle. The study drug was injected lateral to the artery once the needle tip was visualized and after careful negative blood aspiration incremental to sonography to observe expansion of the adductor canal.
The sensory block over the anterior part of the thigh was continuously assessed for 30 min after the ACB using a 3-point scale (0 = loss of sensation to light touch [anesthesia]; 1 = loss of sensation to pinprick [analgesia]; 2 = normal sensations) [14]. Additionally, the onset of sensory block (the time from ACB injection until sensory block grade zero) was recorded. The ACB was considered successful if a complete sensory block (sensory score = 0) was achieved within 30 min of ACB injection. ACB failure was defined as no recording of a grade zero sensory block after 30 min, and these cases were excluded from the study.
The sedation score was assessed 30 min after the IV study drug was administered using a modified Ramsay sedation scale (RSS; 1 = awake and alert, with minimal or no cognitive impairment; 2 = awake but tranquil, with purposeful responses to verbal commands at a conversational level; 3 = appears to be asleep, with purposeful response to verbal commands at a conversational level; 4 = appears to be asleep, with purposeful responses requiring louder than conversational level commands, a light glabellar tap, or both; 5 = asleep, sluggish with purposeful responses requiring loud verbal commands, a strong glabellar tap, or both; 6 = asleep, with sluggish purposeful responses only to painful stimuli; 7 = asleep, with reflex withdrawal to painful stimuli only; and 8 = unresponsive to external stimuli, including pain) [15].
Spinal anesthesia was administered under sterile conditions with the patient in a sitting position. Intrathecal administration of 12.5 or 15 mg of 0.5% hyperbaric bupivacaine was injected according to the patient’s height (height < 165 cm, 12.5 mg; height ≥ 165 cm, 15 mg) at the L3–4 interspace (or one space above or below) with a 22-gauge needle after local skin infiltration with 1% lidocaine. Immediately after spinal anesthesia, the patient was turned to the supine position, and once the spinal sensory level reached T10 (evaluated by a sharp needle), the patient was placed in a slight semi-sitting position and remained in that position throughout the operation. The patient was kept warm using forced-air warming blankets. Surgery was initiated following tourniquet inflation.
Intraoperatively, the HR and MBP were recorded every 15 min for the first 2 h and every 30 min thereafter. In the event of bradycardia (HR < 50 beats/min), IV atropine (0.5 mg) was administered. Hypotension (decrease in MAP > 20% from baseline) was treated with increments of 5 mg IV ephedrine.
Both the sedation score and shivering grade were assessed 1 h after spinal anesthesia. Shivering was assessed using a 5-point scale (0 = no shivering, 1 = piloerection or peripheral vasoconstriction but no visible shivering, 2 = muscular activity in only one muscle group, 3 = muscular activity in more than one muscle group but not generalized, and 4 = shivering involving the whole body) [16]. A shivering grade ≥ 3 was treated with 0.2 mg/kg IV ketamine.
All patients received 75 mg intramuscular diclofenac sodium after surgery and every 12 h thereafter. Patients were transferred to the recovery room for postoperative monitoring after the surgical duration was recorded. The sedation score was assessed immediately postoperatively, whereas both the sedation and shivering scores were evaluated 1 h after surgery. Postoperative pain (at rest) was evaluated at 2, 4, 8, 12, and 24 h postoperatively, while pain during movement (45-degree active knee flexion) was assessed 24 h postoperatively. All patients with a VAS score ≥ 40 received IV meperidine (0.3 mg/kg) as a rescue analgesic. The analgesic duration (the time from the ACB injection until the first postoperative rescue analgesic), frequency of rescue analgesia, and total cumulative doses of meperidine and ketamine in the first 24 h were recorded.
Data entry and analyses were performed using the Statistical Package for the Social Sciences version 21 (SPSS, Inc., USA). Data were assessed for normal distribution using the Shapiro-Wilk test. Continuous parametric data are presented as the mean ± standard deviation (SD), whereas non-parametric data are presented as the median (interquartile range) or median (minimum – maximum). The Student’s t-test was used to compare the two groups, and the paired t-test was used to compare two measurements within a single group. The chi-squared test was used to analyze categorical data. Statistical significance was set at P < 0.05.
In a previous study conducted by Crowley and Buggy, the incidence of post-spinal shivering was 55%, and a 25% decrease in the incidence of post-spinal shivering (13.75%) was considered clinically significant for detecting clinical effects [17]. An α error of 0.05, β error of 0.2, and study power of 80% revealed a total sample size of 51 patients. Allowing for a 10% dropout, a total of 56 patients (28 in each group) was calculated.
A total of 70 patients were assessed for eligibility, 14 of which were excluded (6 patients for refusal, 4 for not meeting the inclusion criteria, and 4 for failed ACB). A total of 56 patients (28 patients in each group) were thus randomized and underwent unilateral TKA under spinal anesthesia. The patients either received IV dexmedetomidine followed by an ACB with levobupivacaine and saline (IV dex group) or IV saline followed by an ACB with levobupivacaine and dexmedetomidine (ACB dex group) (Fig. 1).
The demographic and surgical characteristics of the patients did not differ significantly between the two groups (Table 1).
The onset of complete sensory block after the ACB was significantly later in the IV dex group (22.14 ± 2.52 min) than in the ACB dex group (12 ± 3.31 min) (P < 0.001) (Table 2).
The incidence of shivering 1 h after spinal anesthesia (primary outcome) was equal in both groups (50%), and neither group required intraoperative ketamine. The shivering incidence and scores were significantly lower in the IV dex group, with no significant change in the number of patients who needed ketamine treatment 1 h postoperatively (Table 3).
Both groups showed a significant decrease in HR values intraoperatively compared to preoperative baseline values at 15, 30, 45, 60, 75, 90, and 105 min (P < 0.001 in both groups) and at 120 min (ACB dex group, P < 0.001; IV dex group, P = 0.012) (Fig. 2A). None of the patients developed intraoperative bradycardia, and no significant differences in HR values were found between the two groups. However, a significant decrease in intraoperative MAP values compared to baseline was observed in both groups at 15, 30, 45, 60, 75, 90, and 105 min and within the IV dex group at 120 and 150 min (Fig. 2B). However, no significant difference in the MAP scores between the two groups was found. Four patients developed hypotension (two in each group).
Compared to the ACB dex group, a significantly greater increase in the sedation score values was seen in the IV dex group preoperatively (0.5 h after administration of the IV study drug), intraoperatively (1 h after spinal anesthesia), and immediately postoperatively (Table 2).
Regarding pain scores (Fig. 3), a statistically significant increase in the VAS scores at rest was found in the IV dex group at 8, 12, and 24 h compared to the ACB dex group (P = 0.030, P = 0.002, and P < 0.001, respectively). Additionally, a significantly higher pain score was detected in the IV dex group during movement 24 h postoperatively than in the ACB dex group (P < 0.001).
The duration of postoperative analgesia (h) was significantly greater in the ACB dex group (12.28 ± 4.47) than in the IV dex group (9.28 ± 1.90) (P = 0.002). Both the frequency of analgesic requirement and the mean meperidine consumption were significantly lower in the ACB dex group (2.14 ± 0.59 and 53.70 ± 14.38 mg, respectively) than in the IV dex group (2.67 ± 0.47 and 67.09 ± 13.96 mg, respectively) during the first postoperative day (Table 2).
The present study demonstrated that the incidence of intraoperative shivering was equal in the IV dex and ABC dex groups; however, the IV dex group had significantly lower postoperative shivering scores.
A previous study conducted by Usta et al. [6] evaluated the effect that IV loading and maintenance of dexmedetomidine infusion had on the prevention of shivering after spinal anesthesia. The incidence of shivering decreased from 56.7% (placebo group) to 10% (P = 0.001). Additionally, Elvan et al. [18] revealed that the incidence of post-anesthesia shivering following 1 µg/kg dexmedetomidine administered over 10 min followed by a continuous infusion at 0.4 µg/kg/h was 17.5%. The lower shivering incidence in these two studies compared to the IV dex group in the current study could be explained by the additional administration of maintenance dexmedetomidine throughout the intraoperative period.
Abdel-Ghaffar et al. [19] revealed that post-spinal shivering is treated effectively in 90% of patients who receive 0.5 µg/kg of IV dexmedetomidine. Likewise, the addition of dexmedetomidine as an adjuvant to the local anesthetic perineurally reduces the incidence of shivering [20]. Hanoura et al. [21] also revealed that the addition of 1 µg/kg dexmedetomidine to an epidural injection in combined spinal-epidural anesthesia in females undergoing elective cesarean section resulted in a significantly decreased incidence of shivering (P = 0.03).
Dexmedetomidine has been documented to reduce shivering by decreasing the thresholds for shivering and vasoconstriction [22]. When dexmedetomidine is administered perineurally, it is absorbed and systemically redistributed, thereby exerting systemically mediated effects [23]. In 2014, Fritsch et al. [24] measured the plasma levels of dexmedetomidine after perineural administration of 150 µg dexmedetomidine in an interscalene nerve block and found that levels peaked 30 min after administration and reduced to very low levels 3h later. Based on the findings of Fritsch et al., the low incidence of shivering in the ACB dex group 1 h after spinal anesthesia (equivalent to 90 min after perineural administration of dexmedetomidine) can be explained by the fact that it was near the time of peaked plasma levels of systemically absorbed dexmedetomidine from the ACB. In contrast, the higher incidence of shivering at 1 h postoperatively (nearly 205 min [3.4 h] after perineural administration of dexmedetomidine) can be explained by the very low plasma levels of systemically absorbed dexmedetomidine from the ACB. Therefore, the superior control of shivering found in the IV dex group could be explained by the longer mean terminal half-life of IV dexmedetomidine, which was found to be 385 ± 144 min (almost 6.4 ± 2.4 h) after administration of 2 µg/kg IV dexmedetomidine [25].
For this study, ketamine was used to treat shivering based on a study conducted by Shakya et al. [26], which compared the effectiveness of low-dose ketamine (0.25 mg/kg) and ondansetron (4 mg) on shivering prevention during spinal anesthesia and revealed that the shivering rate was 4.33 times lower in the ketamine group. Additionally, a small dose of ketamine was used to avoid undesirable side effects such as excessive sedation, hallucinations, nausea, and vomiting. Ketamine is an N-methyl-D-aspartate receptor antagonist that likely controls shivering through non-shivering thermogenesis, influencing either the hypothalamus or the beta-adrenergic effect of norepinephrine [27].
IV dexmedetomidine has been used extensively for anxiolysis and sedation (even as the sole sedative agent); however, preoperative IV dexmedetomidine has been documented to attenuate the stress response associated with anesthesia and surgery and decrease postoperative pain and opioid requirement by up to 90% [28]. In the study conducted by Parikh et al. [29], the dexmedetomidine dose was almost double that used in the IV dex group in the current study (1 µg/kg over 10 min followed by a continuous infusion at 0.2 µg/kg/h) in 45 patients who underwent tympanoplasty under local anesthesia. The authors reported a significant intraoperative reduction in HR (15%–20%) 2 min after the dexmedetomidine bolus had finished (maximum decrease after 10 min) that remained until the end of the surgery. This finding is similar to the significant reduction in HR, mostly over the intraoperative duration, seen in the IV dex group in the current study. Similarly, Parikh et al. [29] reported a significant intraoperative decrease in the MAP (10%–15%) for > 60 min. However, only one patient developed hypotension and bradycardia after receiving the loading infusion, which is consistent with the results of the current study.
Another study revealed that IV dexmedetomidine (1 µg/kg) administered 10 min before tourniquet inflation during a regional blockade decreased the associated HR and MAP from 16% to 20% [30]. This reduction in the HR and MAP after IV dexmedetomidine administration has also been reported in other previous studies [31,32]. This attenuation of hemodynamics following IV dexmedetomidine administration can be explained by the prominent reduction in sympathetic activity [33].
A previous meta-analysis evaluating the effect of perineural dexmedetomidine as an adjuvant to local anesthetics for brachial plexus blockades found a significant incidence of intraoperative bradycardia in some studies; however, the bradycardia was transient and reversible with the administration of IV atropine [34]. Koraki et al. [35] administered 100 µg of dexmedetomidine as an adjuvant to an axillary plexus block that was associated with transient bradycardia; however, hypotension occurred in only three patients. This is consistent with the findings in the ACB dex group in the current study. However, due to the sympatholytic effect following spinal anesthesia in both groups in this study, other associated causes of the reduction in HR and MAP cannot be ruled out [36].
The analgesic properties of IV dexmedetomidine and its opiate-sparing effects have been confirmed under general anesthesia [37]. Parikh et al. [29] revealed that 11.1% of patients required post-tympanoplasty rescue analgesia following 1 µg/kg of IV dexmedetomidine, with low frequency (four patients required one dose and one patient required three doses of fentanyl). This is relatively analogous to the frequency of analgesic requirement in the IV dex group in the current study (2.14 ± 0.59 times), considering the greater invasiveness of TKA surgery. Similarly, Jaakola [30] and Karaaslan et al. [32] have reported significantly lower rescue opioid requirements after 1 µg/kg of IV dexmedetomidine (followed by a continuous infusion at 0.5 µg/kg/h) during nasal septoplasty and hand surgery. Two previous studies used the same volume of levobupivacaine in their ACB groups as that in the ACB dex group in the current study to determine the analgesic effect. One study conducted by Kampitak et al. [38] used 0.5% levobupivacaine alone, while the study by AbdelRady et al. [39] used 0.25% levobupivacaine and 0.5 µg/kg dexmedetomidine. Kampitak et al. administered postoperative ACBs with double the levobupivacaine concentration (20 ml of 0.5% levobupivacaine) but revealed an analgesic duration of almost 2.3 h, which was much shorter than that in the present study. This could be explained by the absence of dexmedetomidine or other adjuvants.
AbdelRady et al. [39], who used the same concentration of levobupivacaine and dexmedetomidine for the ACB, reported a slightly closer analgesic duration (8.5 ± 0.46 h) during the first postoperative 24 h. Additionally, their recorded VAS scores were highest at 6, 8, and 12 h, which is consistent with the findings of the current study, where VAS was highest at 12 and 24 h postoperatively in the ACB dex group. The relatively higher analgesic duration in our study may be related to our confirmation of a successful ACB before spinal anesthesia. The perineural analgesic effect of dexmedetomidine results from the suppression of C-fiber discharge and reduction in inflammatory mediator release [40]. Additionally, dexmedetomidine inhibits the release of substance P from the dorsal horn of the spinal cord, leading to primary analgesic effects [41].
The present study revealed that both groups showed a decrease in the RSS score over time postoperatively. This is consistent with the studies conducted by AbdelRady et al. [39] on ACB dexmedetomidine and Parikh et al. [29] on IV dexmedetomidine, in which no patient had an RSS score > 3. Moreover, Basar et al. [42] demonstrated that the administration of 0.5 µg/kg dexmedetomidine preoperatively resulted in significant sedation with no change in the time of awakening, according to Aldrete’s recovery score. The sedative properties of dexmedetomidine are well-documented and are thought to be primarily mediated by postsynaptic Alpha-2 adrenergic receptors, which differ depending on the receptor [37]. In the current study, assessing the sedative effect in a range from full consciousness to calmness is preferable because patients undergoing TKA are mostly elderly and only 0.5 µg/kg dexmedetomidine was administered to allow for early ambulation and discharge. Additionally, the ACB was performed preoperatively so the patients could benefit from the shivering prevention effect of dexmedetomidine and to allow for the success of the block to be assessed without the masking effect of spinal anesthesia.
One limitation of this study is the absence data on the core temperature, spinal sensory block duration, and respiratory rate.
In conclusion, perineural ACB dexmedetomidine had similar sedative and intraoperative anti-shivering effects as IV dexmedetomidine in patients undergoing TKA under spinal anesthesia. However, postoperatively, ACB dexmedetomidine was associated with lower shivering control but superior analgesia. Further studies on the effect of neuraxial dexmedetomidine on shivering are needed to clarify these findings.
Notes
References
1. Jenstrup MT, Jæger P, Lund J, Fomsgaard JS, Bache S, Mathiesen O, et al. Effects of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand. 2012; 56:357–64.
2. Jæger P, Grevstad U, Henningsen MH, Gottschau B, Mathiesen O, Dahl JB. Effect of adductor-canal-blockade on established, severe post-operative pain after total knee arthroplasty: a randomised study. Acta Anaesthesiol Scand. 2012; 56:1013–9.
3. Lund J, Jenstrup MT, Jæger P, Sørensen AM, Dahl JB. Continuous adductor-canal-blockade for adjuvant post-operative analgesia after major knee surgery: preliminary results. Acta Anaesthesiol Scand. 2011; 55:14–9.
4. Jaeger P, Nielsen ZJ, Henningsen MH, Hilsted KL, Mathiesen O, Dahl JB. Adductor canal block versus femoral nerve block and quadriceps strength: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Anesthesiology. 2013; 118:409–15.
5. Kwofie MK, Shastri UD, Gadsden JC, Sinha SK, Abrams JH, Xu D, et al. The effects of ultrasound-guided adductor canal block versus femoral nerve block on quadriceps strength and fall risk: a blinded, randomized trial of volunteers. Reg Anesth Pain Med. 2013; 38:321–5.
6. Usta B, Gozdemir M, Demircioglu RI, Muslu B, Sert H, Yaldiz A. Dexmedetomidine for the prevention of shivering during spinal anesthesia. Clinics (Sao Paulo). 2011; 66:1187–91.
7. Park SM, Mangat HS, Berger K, Rosengart AJ. Efficacy spectrum of antishivering medications: meta-analysis of randomized controlled trials. Crit Care Med. 2012; 40:3070–82.
8. Liu ZX, Xu FY, Liang X, Zhou M, Wu L, Wu JR, et al. Efficacy of dexmedetomidine on postoperative shivering: a meta-analysis of clinical trials. Can J Anaesth. 2015; 62:816–29.
9. Abdallah FW, Abrishami A, Brull R. The facilitatory effects of intravenous dexmedetomidine on the duration of spinal anesthesia: a systematic review and meta-analysis. Anesth Analg. 2013; 117:271–8.
10. Mahendru V, Tewari A, Katyal S, Grewal A, Singh MR, Katyal R. A comparison of intrathecal dexmedetomidine, clonidine, and fentanyl as adjuvants to hyperbaric bupivacaine for lower limb surgery: a double blind controlled study. J Anaesthesiol Clin Pharmacol. 2013; 29:496–502.
11. Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: a volunteer study. Br J Anaesth. 2013; 110:438–42.
12. Cimen ZS, Hanci A, Sivrikaya GU, Kilinc LT, Erol MK. Comparison of buccal and nasal dexmedetomidine premedication for pediatric patients. Paediatr Anaesth. 2013; 23:134–8.
13. Karaaslan D, Peker TT, Alaca A, Ozmen S, Kirdemir P, Yorgancigil H, et al. Comparison of buccal and intramuscular dexmedetomidine premedication for arthroscopic knee surgery. J Clin Anesth. 2006; 18:589–93.
14. Parrington SJ, O’Donnell D, Chan VW, Brown-Shreves D, Subramanyam R, Qu M, et al. Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. Reg Anesth Pain Med. 2010; 35:422–6.
15. Gill M, Green SM, Krauss B. A study of the Bispectral Index Monitor during procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2003; 41:234–41.
16. Tsai YC, Chu KS. A comparison of tramadol, amitriptyline, and meperidine for postepidural anesthetic shivering in parturients. Anesth Analg. 2001; 93:1288–92.
17. Crowley LJ, Buggy DJ. Shivering and neuraxial anesthesia. Reg Anesth Pain Med. 2008; 33:241–52.
18. Elvan EG, Oç B, Uzun S, Karabulut E, Coşkun F, Aypar U. Dexmedetomidine and postoperative shivering in patients undergoing elective abdominal hysterectomy. Eur J Anaesthesiol. 2008; 25:357–64.
19. Abdel-Ghaffar HS, Mohamed SA, Fares KM, Osman MA. Safety and efficacy of dexmedetomidine in treating post spinal anesthesia shivering: a randomized clinically controlled dose-finding trial. Pain Physician. 2016; 19:243–53.
20. Suresh S, Ecoffey C, Bosenberg A, Lonnqvist PA, de Oliveira GS Jr, de Leon Casasola O, et al. The European Society of Regional Anaesthesia and Pain Therapy/American Society of Regional Anesthesia and Pain Medicine recommendations on local anesthetics and adjuvants dosage in pediatric regional anesthesia. Reg Anesth Pain Med. 2018; 43:211–6.
21. Hanoura SE, Hassanin R, Singh R. Intraoperative conditions and quality of postoperative analgesia after adding dexmedetomidine to epidural bupivacaine and fentanyl in elective cesarean section using combined spinal-epidural anesthesia. Anesth Essays Res. 2013; 7:168–72.
22. Talke P, Tayefeh F, Sessler DI, Jeffrey R, Noursalehi M, Richardson C. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. Anesthesiology. 1997; 87:835–41.
23. Andersen JH, Grevstad U, Siegel H, Dahl JB, Mathiesen O, Jæger P. Does dexmedetomidine have a perineural mechanism of action when used as an adjuvant to ropivacaine?: A paired, blinded, randomized trial in healthy volunteers. Anesthesiology. 2017; 126:66–73.
24. Fritsch G, Danninger T, Allerberger K, Tsodikov A, Felder TK, Kapeller M, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014; 39:37–47.
25. Dyck JB, Maze M, Haack C, Vuorilehto L, Shafer SL. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993; 78:813–20.
26. Shakya S, Chaturvedi A, Sah BP. Prophylactic low dose ketamine and ondansetron for prevention of shivering during spinal anaesthesia. J Anaesthesiol Clin Pharmacol. 2010; 26:465–9.
27. Dal D, Kose A, Honca M, Akinci SB, Basgul E, Aypar U. Efficacy of prophylactic ketamine in preventing postoperative shivering. Br J Anaesth. 2005; 95:189–92.
28. Aho M, Erkola O, Kallio A, Scheinin H, Korttila K. Dexmedetomidine infusion for maintenance of anesthesia in patients undergoing abdominal hysterectomy. Anesth Analg. 1992; 75:940–6.
29. Parikh DA, Kolli SN, Karnik HS, Lele SS, Tendolkar BA. A prospective randomized double-blind study comparing dexmedetomidine vs. combination of midazolam-fentanyl for tympanoplasty surgery under monitored anesthesia care. J Anaesthesiol Clin Pharmacol. 2013; 29:173–8.
30. Jaakola ML. Dexmedetomidine premedication before intravenous regional anesthesia in minor outpatient hand surgery. J Clin Anesth. 1994; 6:204–11.
31. Na HS, Song IA, Park HS, Hwang JW, Do SH, Kim CS. Dexmedetomidine is effective for monitored anesthesia care in outpatients undergoing cataract surgery. Korean J Anesthesiol. 2011; 61:453–9.
32. Karaaslan K, Yilmaz F, Gulcu N, Colak C, Sereflican M, Kocoglu H. Comparison of dexmedetomidine and midazolam for monitored anesthesia care combined with tramadol via patient-controlled analgesia in endoscopic nasal surgery: a prospective, randomized, double-blind, clinical study. Curr Ther Res Clin Exp. 2007; 68:69–81.
33. Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000; 93:1345–9.
34. Hussain N, Grzywacz VP, Ferreri CA, Atrey A, Banfield L, Shaparin N, et al. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block: a systematic review and meta-analysis of 18 randomized controlled trials. Reg Anesth Pain Med. 2017; 42:184–96.
35. Koraki E, Stachtari C, Kapsokalyvas I, Stergiouda Z, Katsanevaki A, Trikoupi A. Dexmedetomidine as an adjuvant to 0.5% ropivacaine in ultrasound-guided axillary brachial plexus block. J Clin Pharm Ther. 2018; 43:348–52.
36. Gao L, Zheng G, Han J, Wang Y, Zheng J. Effects of prophylactic ondansetron on spinal anesthesia-induced hypotension: a meta-analysis. Int J Obstet Anesth. 2015; 24:335–43.
37. Smith H, Elliott J. Alpha(2) receptors and agonists in pain management. Curr Opin Anaesthesiol. 2001; 14:513–8.
38. Kampitak W, Tanavalee A, Ngarmukos S, Amarase C, Apihansakorn R, Vorapalux P. Does adductor canal block have a synergistic effect with local infiltration analgesia for enhancing ambulation and improving analgesia after total knee arthroplasty? Knee Surg Relat Res. 2018; 30:133–41.
39. AbdelRady MM, Ali WN, Younes KT, Talaat EA, AboElfadl GM. Analgesic efficacy of single-shot adductor canal block with levobupivacaine and dexmedetomidine in total knee arthroplasty: a randomized clinical trial. Egypt J Anaesth. 2021; 37:386–93.
40. Memary E, Mirkheshti A, Dabbagh A, Taheri M, Khadempour A, Shirian S. The effect of perineural administration of dexmedetomidine on narcotic consumption and pain intensity in patients undergoing femoral shaft fracture surgery; a randomized single-blind clinical trial. Chonnam Med J. 2017; 53:127–32.
41. Venn RM, Bryant A, Hall GM, Grounds RM. Effects of dexmedetomidine on adrenocortical function, and the cardiovascular, endocrine and inflammatory responses in post-operative patients needing sedation in the intensive care unit. Br J Anaesth. 2001; 86:650–6.
42. Basar H, Akpinar S, Doganci N, Buyukkocak U, Kaymak Ç, Sert O, et al. The effects of preanesthetic, single-dose dexmedetomidine on induction, hemodynamic, and cardiovascular parameters. J Clin Anesth. 2008; 20:431–6.
Fig. 1.
CONSORT flow diagram of participant selection. IV dex: intravenous dexmedetomidine, ACB dex: adductor canal blockade dexmedetomidine.
Fig. 2.
(A) Heart rate (beats/min) of the study groups. (B) Mean arterial pressure (mmHg) of the study groups. Values are presented as mean ± SD. IV dex: intravenous dexmedetomidine, ACB dex: adductor canal blockade dexmedetomidine. *Statistically significant difference (P < 0.05) with baseline values within the IV dex group. †Statistically significant difference (P < 0.05) with baseline values within the ACB dex group.
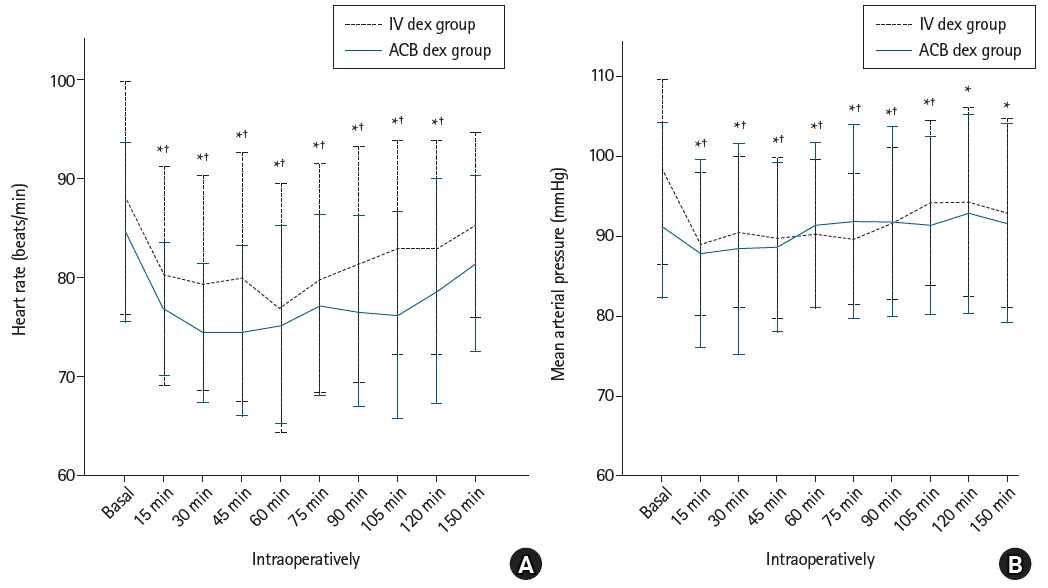
Fig. 3.
Pain scores of the study groups. Values are presented as mean ± SD. IV dex: intravenous dexmedetomidine, ACB dex: adductor canal blockade dexmedetomidine. *Significant difference (P < 0.05) between the two groups.
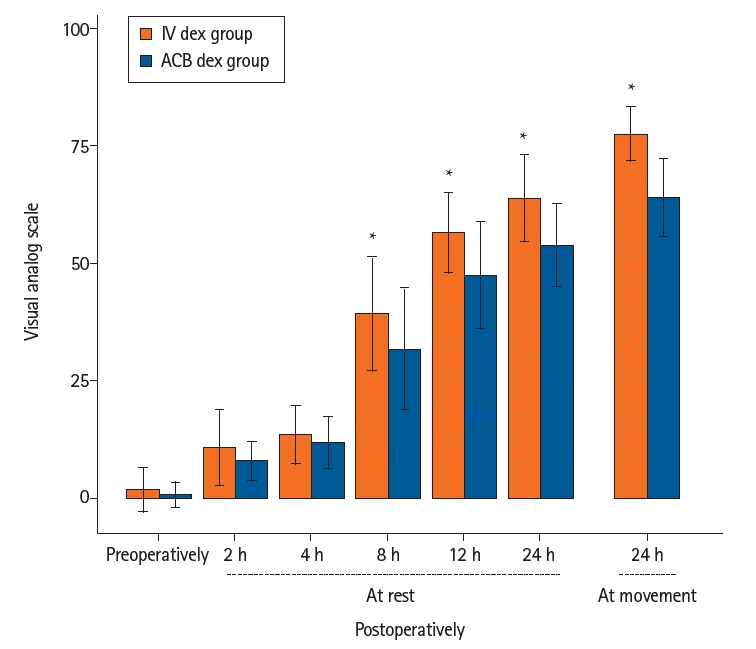
Table 1.
Demographic and Surgical Data according to Study Group
Table 2.
Sensory Block, Sedation Score, and Analgesic Profile according to Study Group
Table 3.
Shivering Profile and Ketamine Dosage according to Study Group




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download