1. Löser C, Aschl G, Hébuterne X, et al. ESPEN guidelines on artificial enteral nutrition: percutaneous endoscopic gastrostomy (PEG). Clin Nutr. 2005; 24:848–861.
2. Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980; 15:872–875.
3. Ponsky JL, Gauderer MW. Percutaneous endoscopic gastrostomy: a nonoperative technique for feeding gastrostomy. Gastrointest Endosc. 1981; 27:9–11.
4. Lee JM, Park Y, Park JM, et al. New sedatives and analgesic drugs for gastrointestinal endoscopic procedures. Clin Endosc. 2022; 55:581–587.
5. Goudra B, Saumoy M. Anesthesia for advanced endoscopic procedures. Clin Endosc. 2022; 55:1–7.
6. Pih GY, Na HK, Hong SK, et al. clinical outcomes of percutaneous endoscopic gastrostomy in the surgical intensive care unit. Clin Endosc. 2020; 53:705–716.
7. ASGE Technology Committee, Kwon RS, Banerjee S, et al. Enteral nutrition access devices. Gastrointest Endosc. 2010; 72:236–248.
8. Hull MA, Rawlings J, Murray FE, et al. Audit of outcome of long-term enteral nutrition by percutaneous endoscopic gastrostomy. Lancet. 1993; 341:869–872.
9. Sarkar P, Cole A, Scolding NJ, et al. Percutaneous endoscopic gastrostomy tube insertion in neurodegenerative disease: a retrospective study and literature review. Clin Endosc. 2017; 50:270–278.
10. ASGE Standards of Practice Committee, Jain R, Maple JT, et al. The role of endoscopy in enteral feeding. Gastrointest Endosc. 2011; 74:7–12.
11. Arvanitakis M, Gkolfakis P, Despott EJ, et al. Endoscopic management of enteral tubes in adult patients. Part 1: Definitions and indications. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021; 53:81–92.
12. Gkolfakis P, Arvanitakis M, Despott EJ, et al. Endoscopic management of enteral tubes in adult patients. Part 2: Peri- and post-procedural management. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021; 53:178–195.
13. Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. Cochrane handbook for systematic reviews of interventions ver. 6.3 [Internet]. Cochrane; 2022 [updated 2022 Feb; cited 2023 Feb 27]. Available from:
www.training.cochrane.org/handbook.
14. Hinneburg I. ROBINS-1: a tool for asssessing risk of bias in non-randomised studies of interventions. Med Monatsschr Pharm. 2017; 40:175–177.
15. Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations [Internet]. [updated 2013 Oct; cited 2023 Feb 27]. Available from:
https://gdt.gradepro.org/app/handbook/handbook.
16. Geeganage C, Beavan J, Ellender S, et al. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database Syst Rev. 2012; 10:CD000323.
17. Goldberg LS, Altman KW. The role of gastrostomy tube placement in advanced dementia with dysphagia: a critical review. Clin Interv Aging. 2014; 9:1733–1739.
18. Lee YF, Hsu TW, Liang CS, et al. The efficacy and safety of tube feeding in advanced dementia patients: a systemic review and meta-analysis study. J Am Med Dir Assoc. 2021; 22:357–363.
19. Grant JP. Comparison of percutaneous endoscopic gastrostomy with Stamm gastrostomy. Ann Surg. 1988; 207:598–603.
20. Grant DG, Bradley PT, Pothier DD, et al. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol. 2009; 34:103–112.
21. Yuan Y, Zhao Y, Xie T, et al. Percutaneous endoscopic gastrostomy versus percutaneous radiological gastrostomy for swallowing disturbances. Cochrane Database Syst Rev. 2016; 2:CD009198.
22. Sampson EL, Candy B, Jones L. Enteral tube feeding for older people with advanced dementia. Cochrane Database Syst Rev. 2009; 2009:CD007209.
23. Strijbos D, Keszthelyi D, Bogie RM, et al. A systematic review and meta-analysis on outcomes and complications of percutaneous endoscopic versus radiologic gastrostomy for enteral feeding. J Clin Gastroenterol. 2018; 52:753–764.
24. Roveron G, Antonini M, Barbierato M, et al. Clinical practice guidelines for the nursing management of percutaneous endoscopic gastrostomy and jejunostomy (PEG/PEJ) in adult patients: an executive summary. J Wound Ostomy Continence Nurs. 2018; 45:326–334.
25. George BP, Kelly AG, Albert GP, et al. Timing of percutaneous endoscopic gastrostomy for acute ischemic stroke: an observational study from the US nationwide inpatient sample. Stroke. 2017; 48:420–427.
26. Malmgren A, Hede GW, Karlström B, et al. Indications for percutaneous endoscopic gastrostomy and survival in old adults. Food Nutr Res. 2011; 55.
27. Bannerman E, Pendlebury J, Phillips F, et al. A cross-sectional and longitudinal study of health-related quality of life after percutaneous gastrostomy. Eur J Gastroenterol Hepatol. 2000; 12:1101–1109.
28. Pih GY, Na HK, Ahn JY, et al. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy insertion. BMC Gastroenterol. 2018; 18:101.
29. Lodin D, Gupta AK, Rubay D, et al. The effectiveness of laparoscopic-assisted percutaneous endoscopic gastrostomy in patients with unfavorable anatomy: a single-center retrospective cohort study. Cureus. 2020; 12:e6647.
30. Bender JS. Percutaneous endoscopic gastrostomy placement in the morbidly obese. Gastrointest Endosc. 1992; 38:97–98.
31. Lucendo AJ, Sánchez-Casanueva T, Redondo O, et al. Risk of bleeding in patients undergoing percutaneous endoscopic gastrotrostomy (PEG) tube insertion under antiplatelet therapy: a systematic review with a meta-analysis. Rev Esp Enferm Dig. 2015; 107:128–136.
32. Moon SY, Jung MK, Heo J. Endoscopic hemostasis using an over-the-scope clip for massive bleeding after percutaneous endoscopic gastrostomy removal: a case report. Clin Endosc. 2022; 55:443–446.
33. Lim H, Gong EJ, Min BH, et al. Clinical practice guideline for the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy. Clin Endosc. 2020; 53:663–677.
34. Wollman B, D'Agostino HB, Walus-Wigle JR, et al. Radiologic, endoscopic, and surgical gastrostomy: an institutional evaluation and meta-analysis of the literature. Radiology. 1995; 197:699–704.
35. Elliott LA, Sheridan MB, Denyer M, et al. PEG: is the E necessary? A comparison of percutaneous and endoscopic gastrostomy. Clin Radiol. 1996; 51:341–344.
36. Bankhead RR, Fisher CA, Rolandelli RH. Gastrostomy tube placement outcomes: comparison of surgical, endoscopic, and laparoscopic methods. Nutr Clin Pract. 2005; 20:607–612.
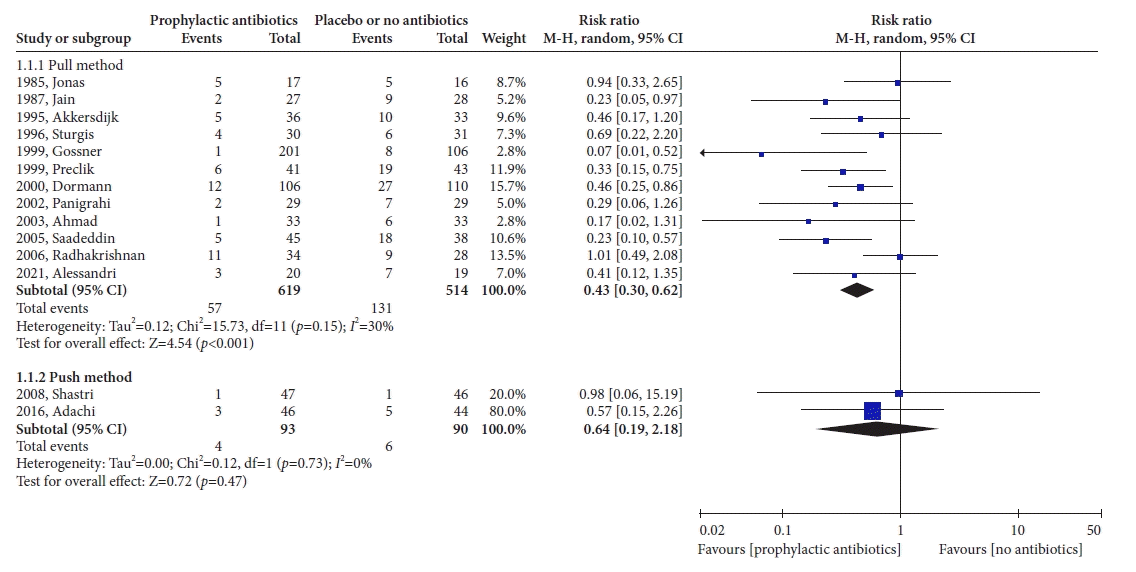
37. Jonas SK, Neimark S, Panwalker AP. Effect of antibiotic prophylaxis in percutaneous endoscopic gastrostomy. Am J Gastroenterol. 1985; 80:438–441.
38. Jain NK, Larson DE, Schroeder KW, et al. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy. A prospective, randomized, double-blind clinical trial. Ann Intern Med. 1987; 107:824–828.
39. Akkersdijk WL, van Bergeijk JD, van Egmond T, et al. Percutaneous endoscopic gastrostomy (PEG): comparison of push and pull methods and evaluation of antibiotic prophylaxis. Endoscopy. 1995; 27:313–316.
40. Sturgis TM, Yancy W, Cole JC, et al. Antibiotic prophylaxis in percutaneous endoscopic gastrostomy. Am J Gastroenterol. 1996; 91:2301–2304.
41. Gossner L, Keymling J, Hahn EG, et al. Antibiotic prophylaxis in percutaneous endoscopic gastrostomy (PEG): a prospective randomized clinical trial. Endoscopy. 1999; 31:119–124.
42. Preclik G, Grüne S, Leser HG, et al. Prospective, randomised, double blind trial of prophylaxis with single dose of co-amoxiclav before percutaneous endoscopic gastrostomy. BMJ. 1999; 319:881–884.
43. Dormann AJ, Wigginghaus B, Risius H, et al. Antibiotic prophylaxis in percutaneous endoscopic gastrostomy (PEG): results from a prospective randomized multicenter trial. Z Gastroenterol. 2000; 38:229–234.
44. Panigrahi H, Shreeve DR, Tan WC, et al. Role of antibiotic prophylaxis for wound infection in percutaneous endoscopic gastrostomy (PEG): result of a prospective double-blind randomized trial. J Hosp Infect. 2002; 50:312–315.
45. Ahmad I, Mouncher A, Abdoolah A, et al. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy: a prospective, randomised, double-blind trial. Aliment Pharmacol Ther. 2003; 18:209–215.
46. Saadeddin A, Freshwater DA, Fisher NC, et al. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy for non-malignant conditions: a double-blind prospective randomized controlled trial. Aliment Pharmacol Ther. 2005; 22:565–570.
47. Radhakrishnan NV, Shenoy AH, Cartmill I, et al. Addition of local antiseptic spray to parenteral antibiotic regimen reduces the incidence of stomal infection following percutaneous endoscopic gastrostomy: a randomized controlled trial. Eur J Gastroenterol Hepatol. 2006; 18:1279–1284.
48. Alessandri F, Strisciuglio C, Borrazzo C, et al. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy in children: a randomised controlled trial. J Pediatr Gastroenterol Nutr. 2021; 72:366–371.
49. Shastri YM, Hoepffner N, Tessmer A, et al. New introducer PEG gastropexy does not require prophylactic antibiotics: multicenter prospective randomized double-blind placebo-controlled study. Gastrointest Endosc. 2008; 67:620–628.
50. Adachi Y, Akino K, Mita H, et al. Systemic prophylactic antibiotics for the modified introducer method for percutaneous endoscopic gastrostomy: a prospective, randomized, double-blind study. J Clin Gastroenterol. 2016; 50:727–732.
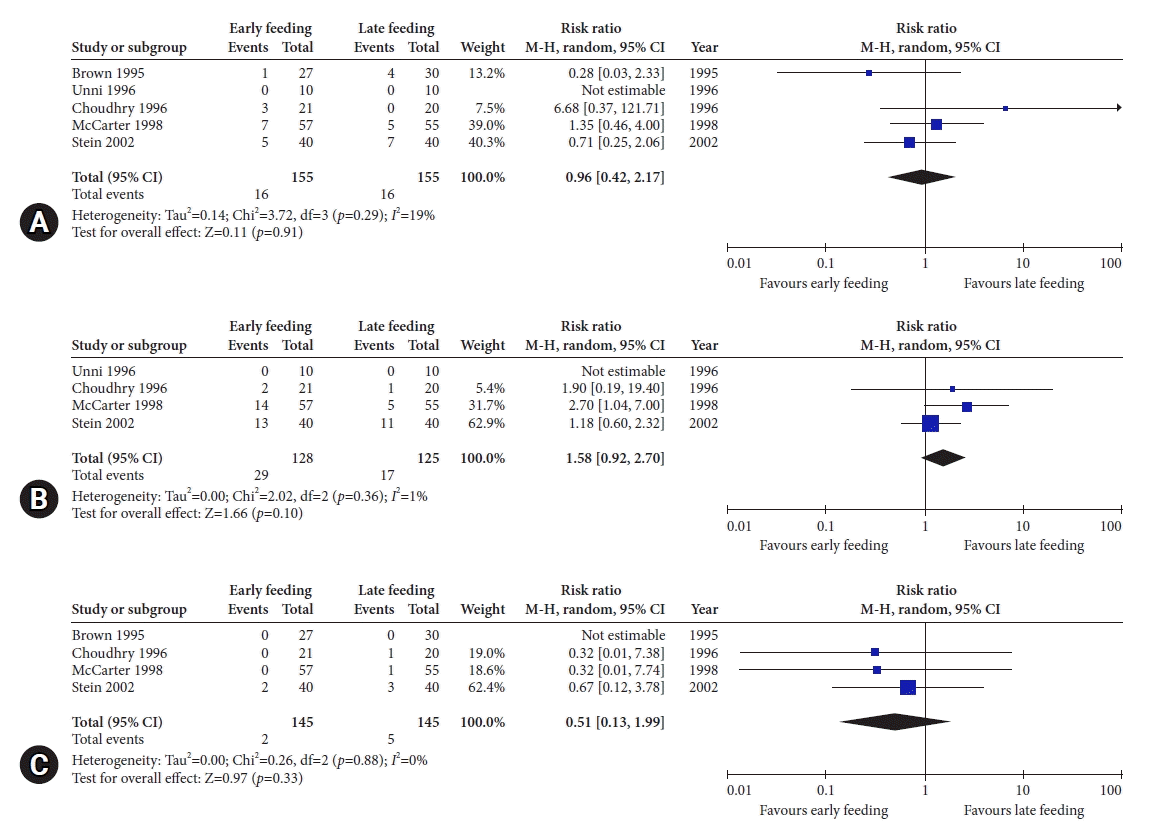
51. Brown DN, Miedema BW, King PD, et al. Safety of early feeding after percutaneous endoscopic gastrostomy. J Clin Gastroenterol. 1995; 21:330–331.
52. Choudhry U, Barde CJ, Markert R, et al. Percutaneous endoscopic gastrostomy: a randomized prospective comparison of early and delayed feeding. Gastrointest Endosc. 1996; 44:164–167.
53. Unni M, Gumaste V, Dave P, Wasserman D. Percutaneous endoscopic gastrostomy (PEG): is it safe to begin feeding in three hours after insertion? Gastrointest Endosc. 1996; 43:360.
54. McCarter TL, Condon SC, Aguilar RC, et al. Randomized prospective trial of early versus delayed feeding after percutaneous endoscopic gastrostomy placement. Am J Gastroenterol. 1998; 93:419–421.
55. Stein J, Schulte-Bockholt A, Sabin M, et al. A randomized prospective trial of immediate vs. next-day feeding after percutaneous endoscopic gastrostomy in intensive care patients. Intensive Care Med. 2002; 28:1656–1660.
56. Lee SW, Lee JH, Cho H, et al. Comparison of clinical outcomes associated with pull-type and introducer-type percutaneous endoscopic gastrostomies. Clin Endosc. 2014; 47:530–537.
57. Campoli PM, de Paula AA, Alves LG, et al. Effect of the introducer technique compared with the pull technique on the peristomal infection rate in PEG: a meta-analysis. Gastrointest Endosc. 2012; 75:988–996.
58. Köhler G, Kalcher V, Koch OO, et al. Comparison of 231 patients receiving either "pull-through" or "push" percutaneous endoscopic gastrostomy. Surg Endosc. 2015; 29:170–175.
59. Maetani I, Tada T, Ukita T, et al. PEG with introducer or pull method: a prospective randomized comparison. Gastrointest Endosc. 2003; 57:837–841.
60. Ohno T, Ogawa A, Yanai M, et al. The usefulness and safety of the introducer technique using a bumper-button-type device as compared with the pull method for percutaneous endoscopic gastrostomy. Surg Laparosc Endosc Percutan Tech. 2015; 25:e1–e4.
61. Retes FA, Kawaguti FS, de Lima MS, et al. Comparison of the pull and introducer percutaneous endoscopic gastrostomy techniques in patients with head and neck cancer. United European Gastroenterol J. 2017; 5:365–373.
62. Sartori S, Trevisani L, Nielsen I, et al. Percutaneous endoscopic gastrostomy placement using the pull-through or push-through techniques: is the second pass of the gastroscope necessary? Endoscopy. 1996; 28:686–688.
63. Tucker AT, Gourin CG, Ghegan MD, et al. Push' versus 'pull' percutaneous endoscopic gastrostomy tube placement in patients with advanced head and neck cancer. Laryngoscope. 2003; 113:1898–1902.
64. Van Dyck E, Macken EJ, Roth B, et al. Safety of pull-type and introducer percutaneous endoscopic gastrostomy tubes in oncology patients: a retrospective analysis. BMC Gastroenterol. 2011; 11:23.
65. Fung E, Strosberg DS, Jones EL, et al. Incidence of abdominal wall metastases following percutaneous endoscopic gastrostomy placement in patients with head and neck cancer. Surg Endosc. 2017; 31:3623–3627.
66. Siu J, Fuller K, Nadler A, et al. Metastasis to gastrostomy sites from upper aerodigestive tract malignancies: a systematic review and meta-analysis. Gastrointest Endosc. 2020; 91:1005–1014.
67. Burney RE, Bryner BS. Safety and long-term outcomes of percutaneous endoscopic gastrostomy in patients with head and neck cancer. Surg Endosc. 2015; 29:3685–3689.
68. Wei MT, Ahn JY, Friedland S. Over-the-scope clip in the treatment of gastrointestinal leaks and perforations. Clin Endosc. 2021; 54:798–804.
69. Macedo C, Almeida N, Alves AR, et al. Persistent peristomal leakage from percutaneous endoscopic gastrostomy successfully treated with argon plasma coagulation. GE Port J Gastroenterol. 2021; 28:210–214.
70. Stanich PP, Sklaw B, Krishna SG. Persistent peristomal leakage from percutaneous endoscopic gastrostomy successfully treated with endoscopic suturing. Endoscopy. 2013; 45 Suppl 2 UCTN:E394.
71. McClave SA, Chang WK. Complications of enteral access. Gastrointest Endos. 2003; 58:739–751.
72. Itkin M, DeLegge MH, Fang JC, et al. Multidisciplinary practical guidelines for gastrointestinal access for enteral nutrition and decompression from the Society of Interventional Radiology and American Gastroenterological Association (AGA) Institute, with endorsement by Canadian Interventional Radiological Association (CIRA) and Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Gastroenterology. 2011; 141:742–765.
73. Westaby D, Young A, O'Toole P, et al. The provision of a percutaneously placed enteral tube feeding service. Gut. 2010; 59:1592–1605.
74. Toussaint E, Van Gossum A, Ballarin A, et al. Enteral access in adults. Clin Nutr. 2015; 34:350–358.
75. Siau K, Troth T, Gibson E, et al. How long do percutaneous endoscopic gastrostomy feeding tubes last? A retrospective analysis. Postgrad Med J. 2018; 94:469–474.
76. Bischoff SC, Austin P, Boeykens K, et al. ESPEN guideline on home enteral nutrition. Clin Nutr. 2020; 39:5–22.
77. Metussin A, Sia R, Bakar S, et al. Foley catheters as temporary gastrostomy tubes: experience of a nurse-led service. Gastroenterol Nurs. 2016; 39:273–277.
78. Schrag SP, Sharma R, Jaik NP, et al. Complications related to percutaneous endoscopic gastrostomy (PEG) tubes. A comprehensive clinical review. J Gastrointestin Liver Dis. 2007; 16:407–418.
79. Anderloni A, Di Leo M, Barzaghi F, et al. Complications and early mortality in percutaneous endoscopic gastrostomy placement in lombardy: a multicenter prospective cohort study. Dig Liver Dis. 2019; 51:1380–1387.
80. El AZ, Arvanitakis M, Ballarin A, et al. Buried bumper syndrome: low incidence and safe endoscopic management. Acta Gastroenterol Belg. 2011; 74:312–316.
81. Cyrany J, Rejchrt S, Kopacova M, et al. Buried bumper syndrome: a complication of percutaneous endoscopic gastrostomy. World J Gastroenterol. 2016; 22:618–627.
82. McClave SA, Jafri NS. Spectrum of morbidity related to bolster placement at time of percutaneous endoscopic gastrostomy: buried bumper syndrome to leakage and peritonitis. Gastrointest Endosc Clin N Am. 2007; 17:731–746.
83. Casper M, Lammert F. How to improve success rates of endoscopic management for buried bumper syndrome. QJM. 2018; 111:467–472.
84. Mueller-Gerbes D, Hartmann B, Lima JP, et al. Comparison of removal techniques in the management of buried bumper syndrome: a retrospective cohort study of 82 patients. Endosc Int Open. 2017; 5:E603–E607.
85. Boeykens K, Duysburgh I. Prevention and management of major complications in percutaneous endoscopic gastrostomy. BMJ Open Gastroenterol. 2021; 8:e000628.
86. Nishiwaki S, Araki H, Fang JC, et al. Retrospective analyses of complications associated with transcutaneous replacement of percutaneous gastrostomy and jejunostomy feeding devices. Gastrointest Endosc. 2011; 74:784–791.
87. Lisotti A, Teci E, Calì A, et al. Percutaneous endoscopic gastrostomy (PEG) tube home replacement: prospective evaluation of a standardized protocol. Endoscopy. 2019; 51:S84.
88. Jo IH, Kim HH, Choi MG, et al. Analysis of risk factors for early tube exchange in percutaneous endoscopic gastrostomy. Korean J Helicobacter Up Gastrointest Res. 2014; 14:261–267.
89. Lee CG, Kang HW, Lim YJ, et al. Comparison of complications between endoscopic and percutaneous replacement of percutaneous endoscopic gastrostomy tubes. J Korean Med Sci. 2013; 28:1781–1787.
90. Sbeit W, Kadah A, Shahin A, et al. Scheduled percutaneous endoscopic gastrostomy tube replacement did not reduce PEG-related complications. Scand J Gastroenterol. 2021; 56:1386–1390.
91. Korula J, Harma C. A simple and inexpensive method of removal or replacement of gastrostomy tubes. JAMA. 1991; 265:1426–1428.
92. Merrick S, Harnden S, Shetty S, et al. An evaluation of the "cut and push" method of percutaneous endoscopic gastrostomy (PEG) removal. JPEN J Parenter Enteral Nutr. 2008; 32:78–80.
93. Pearce CB, Goggin PM, Collett J, et al. The 'cut and push' method of percutaneous endoscopic gastrostomy tube removal. Clin Nutr. 2000; 19:133–135.
94. Kejariwal D, Bromley D, Miao Y. The "cut and push" method of percutaneous endoscopic gastrostomy tube removal in adult patients: the Ipswich experience. Nutr Clin Pract. 2009; 24:281–283.
95. Agha A, AlSaudi D, Furnari M, et al. Feasibility of the cut-and-push method for removing large-caliber soft percutaneous endoscopic gastrostomy devices. Nutr Clin Pract. 2013; 28:490–492.
96. Macchini F, Zanini A, Farris G, et al. Infant percutaneous endoscopic gastrostomy: risks or benefits? Clin Endosc. 2018; 51:260–265.
97. Lim YJ, Yang CH. Technique, management and complications of percutaneous endoscopic gastrostomy. Korean J Gastrointest Endosc. 2009; 39:119–124.
98. Gottfried EB, Plumser AB, Clair MR. Pneumoperitoneum following percutaneous endoscopic gastrostomy. A prospective study. Gastrointest Endosc. 1986; 32:397–399.
99. Allen AI, Vaughan J, Cauthen A, et al. Evaluation of a trial of a desufflation technique to decrease the rate of postoperative pneumoperitoneum after percutaneous endoscopic gastrostomy. Am Surg. 2017; 83:e398–e399.
100. Park WY, Lee TH, Lee JS, et al. Reappraisal of pneumoperitoneum after percutaneous endoscopic gastrostomy. Intest Res. 2015; 13:313–317.
101. Blum CA, Selander C, Ruddy JM, et al. The incidence and clinical significance of pneumoperitoneum after percutaneous endoscopic gastrostomy: a review of 722 cases. Am Surg. 2009; 75:39–43.
102. Nazarian A, Cross W, Kowdley GC. Pneumoperitoneum after percutaneous endoscopic gastrostomy among adults in the intensive care unit: incidence, predictive factors, and clinical significance. Am Surg. 2012; 78:591–594.
103. Murphy CJ, Adler DG, Cox K, et al. Insufflation with carbon dioxide reduces pneumoperitoneum after percutaneous endoscopic gastrostomy (PEG): a randomized controlled trial. Endosc Int Open. 2016; 4:E292–E295.
104. Nishiwaki S, Araki H, Hayashi M, et al. Inhibitory effects of carbon dioxide insufflation on pneumoperitoneum and bowel distension after percutaneous endoscopic gastrostomy. World J Gastroenterol. 2012; 18:3565–3570.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download