Abstract
Background/Aims
Colonoscopy for screening is associated with unpleasant experiences for patients, and abdominal compression devices have been developed to minimize these problems. However, there is a paucity of data supporting the therapeutic benefits of this strategy. This study examined the effects of using an abdominal compression device during colonoscopy on the cecal intubation time (CIT), abdominal compression, patient comfort, and postural changes.
Methods
We searched PubMed and Scopus (from inception to November 2021) for randomized controlled trials that assessed the effects of an abdominal compression device during colonoscopy on CIT, abdominal compression, patient comfort, and postural change. A random-effects meta-analysis was performed. Weighted mean differences (WMDs) and Mantel-Haenszel odds ratios (ORs) were calculated.
Results
Our pooled analysis of seven randomized controlled trials revealed that abdominal compression devices significantly reduced CIT (WMD, –0.76 [–1.49 to –0.03] minutes; p=0.04), abdominal compression (OR, 0.52; 95% confidence interval [CI], 0.28–0.94; p=0.03), and postural changes (OR, 0.46; 95% CI, 0.27–0.78; p=0.004) during colonoscopy. However, our results did not show a significant change in patient comfort (WMD, –0.48; 95% CI, –1.05 to 0.08; p=0.09) when using an abdominal compression device.
Colonoscopy is required for both screening and treatment of colorectal cancer.1-4 In the United States, an estimated 14 million colonoscopies are performed annually, and the use of colonoscopies is increasing globally.5 Despite advances in colonoscopy equipment and personnel training, the procedure can be unpleasant for some people.6
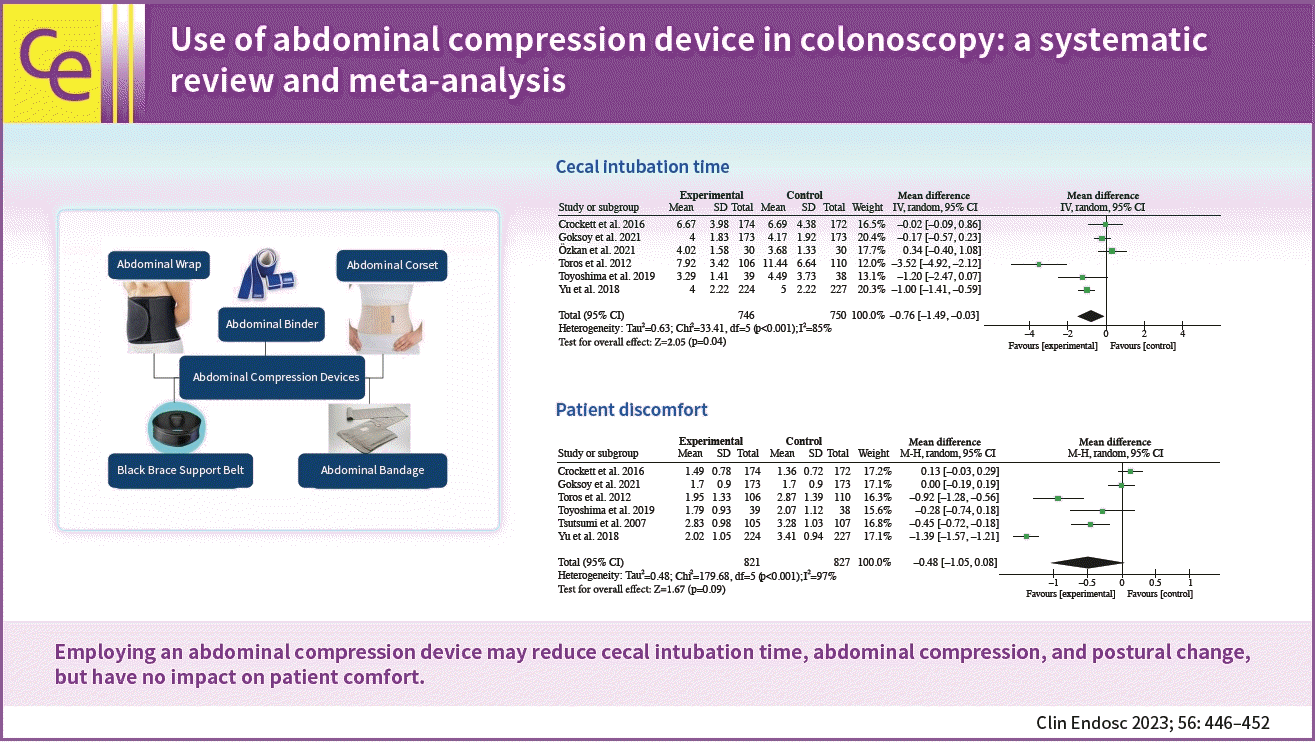
A sigmoid loop is produced during colonoscopy, which may lead to excruciating pain and make endoscope insertion challenging.6 Ancillary maneuvers, such as posture shift and abdominal compression, are regularly employed to prevent looping and pain.7 Abdominal compression can aid in colonoscopy, preventing looping and pain management, as well as improving access to the cecum. Compression of the abdominal cavity during colonoscopy can be accomplished manually (by hand) or with the help of medical instruments that improve compression, such as abdominal compression devices (ACDs) or the abdominal corset.8 An abdominal corset is a bandage wrapped around the abdomen and is often used during abdominal surgery to protect the integrity of the sutures and support the incisional region by providing immobilization.9 Although ACD has been used to reduce cecal intubation time (CIT) and promote patient comfort, no guidelines or data are supporting the therapeutic benefits of this method.
Many randomized controlled trials (RCTs) have investigated the efficacy of encircling an ACD during colonoscopies.5,10-14 Studies have shown that ACD is effective in shortening the time required for cecal intubation during surgery.11,14 However, investigators have concluded that there are discrepancies in their findings.5 Uncertainty exists due to such uneven results when ACD is used in patients undergoing colonoscopy. Hence, we aimed to pool data and conduct a systematic review and meta-analysis of all relevant studies that reported results on abdominal compression, patient comfort, postural changes, and CIT.
This systematic review was performed following Cochrane Handbook for Systematic Reviews of Interventions.12 The study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.15
Two reviewers (AMR and AKS) separately performed a comprehensive search of multiple electronic databases and conference proceedings, including PubMed and Scopus (from inception until November 2021). Supplementary Table 1 lists the search strategies employed for each database. All the selected articles were imported into EndNote X9 (Thomson Reuters) to identify and delete duplicates. The remaining studies were examined by two reviewers, AMR and AKS, based on their titles and abstracts. The entire material was rigorously examined against the inclusion and exclusion criteria before articles were selected. The senior author (YZ) helped resolve any discrepancies.
The studies included in the meta-analysis satisfied the following inclusion criteria: (1) study type: RCT; (2) population: patients undergoing colonoscopy; (3) intervention: ACD; (4) comparator: no device; and (5) outcomes: CIT, abdominal compression, postural changes, and patient comfort. Studies that used pillow-type compression devices were excluded. Conference abstracts, case series, case reports, and reviews were excluded.
The first investigator (AMR) extracted the data and the accuracy was double-checked by a second investigator (AKS). Baseline characteristics, outcomes, weighted mean differences (WMDs), Mantel–Haenszel odds ratios (ORs), and 95% confidence intervals (CIs) were determined. Furthermore, using the Cochrane risk-of-bias tool for RCTs, two reviewers evaluated the quality of the RCTs as low, high, or uncertain.
Statistical analysis was performed using RevMan software (Review Manager ver. 5.3.5; The Nordic Cochrane Centre). We calculated the ORs using the random-effects model and the Mantel–Haenszel method and estimated the WMD with a 95% CI using the inverse variance for continuous data. The I2 test was used to analyze the heterogeneity in the results of the studies. An I2 score of 50% indicated a considerable level of heterogeneity.16 We employed funnel plot asymmetry to detect any publication bias in the meta-analysis and Egger’s regression test to quantify funnel plot asymmetry.
The initial search yielded a total of 494 potential articles. After exclusion, seven studies were included in the meta-analysis. A PRISMA flowchart summarizing the study selection process is shown in Figure 1. The total number of participants was 1,708. The average age of participants was 54.9 years. Three studies were conducted in Turkey, whereas two were conducted in Japan, one in China, and one in the United States. The study characteristics are summarized in Table 1.5,7,10-14
Of the seven selected studies, six reported the effect of ACD on abdominal compression (total number of patients, 1,496; events, 512). According to our pooled analysis, the use of ACD was significantly associated with lower abdominal compression during colonoscopy (OR, 0.52; 95% CI, 0.28–0.94; p=0.03; Fig. 2A). There was significant heterogeneity among the included studies (I2=81%, p<0.001).
Six studies reported results for patient comfort (total patients, 1,648). Our pooled analysis demonstrated that the ACD did not significantly affect the patient's comfort level (WMD, –0.48; 95% CI, –1.05 to 0.08; p=0.09; Fig. 2B). There was significant heterogeneity among the included studies (I2=97%, p<0.001).
Of the seven selected studies, six reported postural changes (total patients, 1,496; events, 400). Our pooled analysis demonstrated that the use of an ACD was associated with a reduction in postural changes (OR, 0.46; 95% CI, 0.27–0.78; p=0.004; Fig. 2C). Significant heterogeneity existed between the studies (I2=59%, p=0.03).
Of the seven selected studies, six reported CIT (total patients, 1,496). Our pooled analysis shows that ACDs significantly reduced CIT when compared to the control group (WMD, –0.76; 95% CI, –1.49 to –0.03 minutes; p=0.04; Fig. 3). However, significant heterogeneity was noted among the included studies (I2=85%, p<0.001).
Overall, most of the studies were of high quality. All studies reported outcome data; however, most failed to report the blinding of participants and personnel.7,11-14 One study13 had a high risk of performance bias. The results of the quality assessment of the included trials are summarized in Supplementary Figure 1. Publication bias was not assessed as the total number of included trials was less than 10.
Our study demonstrated that ACDs are associated with a significant reduction in CIT as well as abdominal compression and postural changes, but the comfort level does not differ much from the baseline.
ACDs can assist in stabilizing the entire colonoscopy procedure by keeping it aligned and preventing it from looping during colonoscopy, leading to a more comfortable insertion and improved patient comfort. In contrast to manual abdominal pressure or position shifting, ACDs are easy to install and provide effective pressure to aid in the procedure.14 Furthermore, ACDs are reasonably priced and can be reused; therefore, they are not financial strain.11,12
According to our findings, the use of ACDs can significantly reduce the time required for cecal intubation. This was consistent with prior research findings.6 In contrast, some studies have demonstrated the opposite result in terms of reduced CIT.5,12 A reduction in CIT can be an essential element in the identification of cancer because the extension of CIT lowers the rate of adenoma detection.17 Patient-associated characteristics, such as low body mass index (BMI), advanced age, female sex, and personnel experience, have a direct impact on CIT.18 One probable explanation for the shorter CIT in individuals with a higher BMI is that these subjects have higher visceral fat, which provides additional support for the passage of the colonoscope.5
Additionally, our findings demonstrate that the use of ACDs is a significant predictor of reduced abdominal compression during colonoscopy. Furthermore, comparable findings were observed in a previous study;6 however, some studies have claimed that manual compression has the same effect in patients, regardless of the use of an ACD.7,19 Several trials have concluded that medical devices that induce compression are not superior to manual compression. Abdominal devices, such as corsets, can be used to apply pressure to the mesentery, preventing stretching and the formation of sigmoid looping.11 These devices exert well-balanced and effective pressure for the duration of the procedure without assistance.
Our findings also showed that the use of an ACD was strongly associated with reduced postural changes. Our findings corroborate previous evidence.6,11 However, some investigators have found that the use of an ACD does not diminish the requirement for position modifications during intubation or cecal imaging.7 Similarly, two further investigations discovered that the use of a device did not affect the frequency of position changes.5,12 Sedation is crucial during colonoscopy, as it also minimizes the need for position changes throughout the procedure; however, further research is required to assess whether compression devices are more successful in minimizing the frequency of postural adjustments when procedures are performed without sedation.
Furthermore, our data showed that employing an ACD did not affect the patient’s comfort level. This finding is in line with that of a previous study, which indicated that ACDs had no noticeable impact on patient-reported comfort levels.6 Colonoscopy can be extremely uncomfortable, which also causes patients to refrain from undergoing it, resulting in delayed diagnosis and treatment. Pain is the most significant contributor to discomfort. In one clinical study, binders were found to greatly minimize the requirement for analgesics during surgery as well as post-operative discomfort,10 but there are still insufficient data to conclude that ACDs are effective in increasing patient comfort.
We updated a previous study6 and provided the most recent information. Our results correspond with those of previous meta-analyses that added two novel outcomes. Furthermore, our findings pave the way for further research on the factors that drive CIT and the effectiveness of individual abdominal devices. Our findings could also aid in revising the colonoscopy guidelines.
This meta-analysis has numerous limitations. Diverse approaches to blinding outcome evaluation and the sedation provided during colonoscopy could be a source of variation. The diversity in endoscopist experience among studies may also be considered a considerable source of heterogeneity, and providing details related to endoscopist experience can help resolve this issue. Experienced endoscopists may often have less difficulty controlling loops, negating any benefits from the devices. Additionally, subgroup analysis was not integrated because the age and BMI cutoff points varied between the studies. Trials with broader and more diverse patient populations in the future will help perform more rigorous analyses.
Our findings show that using an ACD reduces CIT, abdominal compression, and postural change without affecting patient comfort. The therapeutic utility of ACDs (Fig. 4) in clinical practice should be investigated further in the future, with studies focusing on the clinical benefits of these devices.
Supplementary Material
Supplementary Fig. 1. Summary of quality assessment of the included trials.
Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2022.304.
Notes
Author Contributions
Conceptualization: RK, YZ, JSK; Data curation: RK, YZ, JSK; Formal analysis: YZ, AKS, SSJ, AMR, AZ, JSK, RK; Methodology: YZ, AKS, SSJ, AMR, AZ, JSK, RK; Project administration: RK, YZ, JSK; Software: YZ, AKS, SSJ, AMR, AZ, JSK, RK; Supervision: RK, JSK, YZ; Validation: RK, YZ, JSK; Visualization: RK, AZI, YZ, JSK; Writing–original draft: all authors; Writing–review & editing: AKS, RK, AMR.
REFERENCES
1. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014; 370:1298–1306.
2. Nishizawa T, Suzuki H, Takahashi M, et al. Trainee participation during colonoscopy adversely affects polyp and adenoma detection rates. Digestion. 2011; 84:245–246.
3. Ignjatovic A, East JE, Suzuki N, et al. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol. 2009; 10:1171–1178.
4. Ansa BE, Coughlin SS, Alema-Mensah E, et al. Evaluation of colorectal cancer incidence trends in the United States (2000-2014). J Clin Med. 2018; 7:22.
5. Crockett SD, Cirri HO, Kelapure R, et al. Use of an abdominal compression device in colonoscopy: a randomized, sham-controlled trial. Clin Gastroenterol Hepatol. 2016; 14:850–857.
6. Nishizawa T, Suzuki H, Higuchi H, et al. Effects of encircled abdominal compression device in colonoscopy: a meta-analysis. J Clin Med. 2019; 9:11.
7. Özkan ZK, Fındık ÜY, Albayrak D. The impact of wearing an abdominal corset to achieve compression on colonoscopy outcomes: a randomised controlled trial. Gastrointest Nurs. 2021; 19(Sup2):S18–S23.
8. Prechel JA, Young CJ, Hucke R, et al. The importance of abdominal pressure during colonoscopy: techniques to assist the physician and to minimize injury to the patient and assistant. Gastroenterol Nurs. 2005; 28:232–236.
9. Rothman JP, Gunnarsson U, Bisgaard T. Abdominal binders may reduce pain and improve physical function after major abdominal surgery: a systematic review. Dan Med J. 2014; 61:A4941.
10. Goksoy B, Kiyak M. The effectiveness of using an abdominal binder during colonoscopy: a randomized, double-blind, sham-controlled trial. Scand J Gastroenterol. 2021; 56:990–997.
11. Toros AB, Ersoz F, Ozcan O. Does a fitted abdominal corset makes colonoscopy more tolerable? Dig Endosc. 2012; 24:164–167.
12. Toyoshima O, Nishizawa T, Sakitani K, et al. Colonoscopy using back brace support belt: a randomized, prospective trial. JGH Open. 2019; 4:441–445.
13. Tsutsumi S, Fukushima H, Kuwano H. Colonoscopy using an abdominal bandage. Hepatogastroenterology. 2007; 54:1983–1984.
14. Yu GQ, Huang XM, Li HY, et al. Use of an abdominal obstetric binder in colonoscopy: a randomized, prospective trial. J Gastroenterol Hepatol. 2018; 33:1365–1369.
15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009; 62:e1–e34.
16. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.
17. von Renteln D, Robertson DJ, Bensen S, et al. Prolonged cecal insertion time is associated with decreased adenoma detection. Gastrointest Endosc. 2017; 85:574–580.
18. Franco DL, Leighton JA, Gurudu SR. Approach to incomplete colonoscopy: new techniques and technologies. Gastroenterol Hepatol (N Y). 2017; 13:476–483.
19. Runge T, Eluri S, Cirri H, et al. The effect of provider experience on efficacy of ColoWrap use during colonoscopy: results from a randomized-controlled trial: 1579. Am J Gastroenterol. 2015; 110:S681.
Fig. 2.
Forest plot of (A) abdominal compression, (B) patient comfort, and (C) postural change. M-H, Mantel–Haenszel; CI, confidence interval; SD, standard deviation.
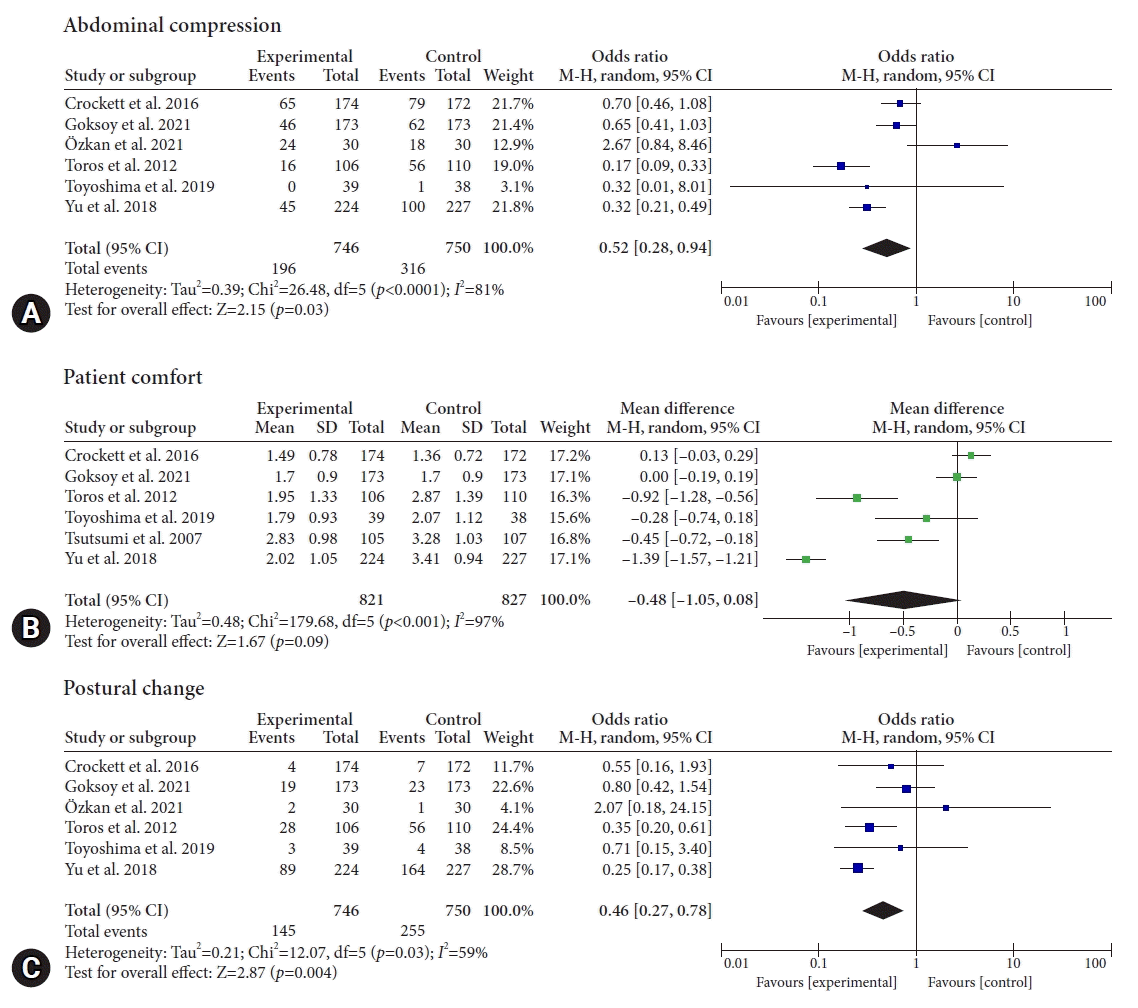
Fig. 3.
Forest plot of cecal intubation time. SD, standard deviation; IV, intravenous; CI, confidence interval.
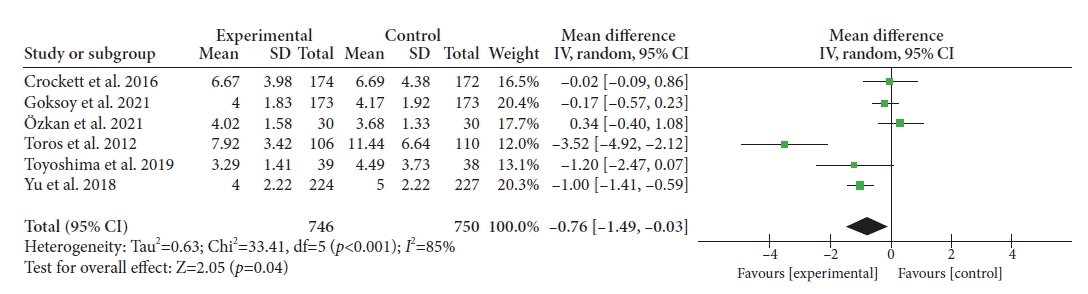
Table 1.
Baseline characteristics of the included studies
| Study | Country | Sample size (n) | Male (n) | Age (yr) | Device used | BMI (kg/m2) | Abdominal circumference (cm) |
|---|---|---|---|---|---|---|---|
| Crockett et al. (2016)5 | USA | 346 | 134 | 59.9±8.7 | Abdominal wrap | 26.6±4.3 | 35.6±4.3 |
| Toros et al. (2012)11 | Turkey | 216 | 97 | 43.1±13.1 | Abdominal corset | 23.7±3.4 | NR |
| Toyoshima et al. (2019)12 | Japan | 77 | 49 | 51.3±10.1 | Black brace support belt | NR | NR |
| Tsutsumi et al. (2007)13 | Japan | 212 | 143 | 67.2 (18–87) | Abdominal bandage | NR | NR |
| Yu et al. (2018)14 | China | 451 | 181 | 54.5±13.4 | Abdominal binder | 24.4±3.9 | NR |
| Goksoy and Kiyak (2021)10 | Turkey | 346 | 141 | 50.5±12.3 | Abdominal binder | 28.8±5.0 | 103±11 |
| Özkan et al. (2021)7 | Turkey | 60 | 29 | 57.8±12.6 | Abdominal corset | 28.0±4.5 | NR |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download