Abstract
Schwannomas exhibit histopathological variation that leads to diagnostic dilemmas, although less frequent in the oral cavity. We describe a case with unique histopathology and no relevant clinical history that adds to the breadth of literature on the diversity presented by Schwannoma. A 60-year-old female patient presented with a small dome-shaped, asymptomatic swelling on the alveolar ridge 6 years in duration. Histopathologically, it showed rich cellular pathology with a unique arrangement of tumor cells forming irregular rosettes. Each rosette presented with a central core of fibrin-collagenous material and the tumor cells were arranged on the periphery, exhibiting epithelioid change with evidence of mild cellular and nuclear pleomorphism. On immunohistochemical evaluation, the cells were strongly and diffusely positive for S-100 and negative for Ki-67. A diagnosis of benign Schwannoma with a rosette-like arrangement with epithelioid change was made. The case report emphasizes the risk of misdiagnosis and the importance of awareness regarding rare histopathological variants of Schwannoma.
Schwannomas are not-so-rare, benign tumors originating from Schwann cells of the nerve sheath1. They are also called neurilemmomas or neurinomas. Twenty-five to forty percent of schwannomas occur in the head and neck region, and only 0.5% to 1% occur in the oral and maxillofacial area1,2. Although the oral cavity has extensive nervous plexuses, it does not correlate with the number of schwannomas that develop there3. The mobile portion of the tongue is a common site, and the mucoperiosteum is less frequently mentioned as a site in the literature2. Schwannomas are predominantly seen in the 3rd to 5th decades of life and affect both sexes in nearly equal numbers4. They have been often reported as asymptomatic, solitary, smooth, and submucosal nodules in the oral cavity2,3. They are mostly sporadic but can be inherited. Either way, they are almost always associated with an inactivation mutation of the neurofibrin-2 gene5.
Under the lens, a schwannoma is an encapsulated tumor of perineurial, well-differentiated Schwann cells that presents a biphasic pattern of Antoni A (richly cellular and well organized) and Antoni B (less cellular and edematous/myxomatous) areas. Schwann cells have long, thin spindle-shaped bodies with indistinct borders and oval, twisted/buckled, or crooked nuclei. These cells appear as organized streaming fascicles in Antoni A, with areas of palisading nuclear arrangements around eosinophilic amorphous masses known as Verocay bodies. On the other hand, Antoni B areas are relatively paucicellular, with haphazardly arranged Schwann cells in a myxoid or loose stroma1,4,6,7. Cystic degeneration, thick hyalinized vessels, hemosiderin deposition, lipid-laden macrophages, and nuclear atypia (degenerative changes) are other features of schwannomas8. Apart from those conventional features, schwannomas can present with many morphological variations and mild cellular and nuclear pleomorphism. Although the conventional histopathology is easily diagnosable, variations can be a source of incorrect interpretation. To the best of our knowledge, schwannoma with analogous histopathology in the oral cavity has rarely been reported in the English-language literature. Therefore, we here present a case of Schwannoma with an extensive rosette-like arrangement of Schwann cells, along with evidence of mild nuclear/cellular pleomorphism and epithelioid cells. Immunohistochemistry can aid in making a definitive diagnosis and ruling out possible malignant behavior in such circumstances. The mainstay of treatment is conservative surgical excision, with no/low recurrences reported2,9.
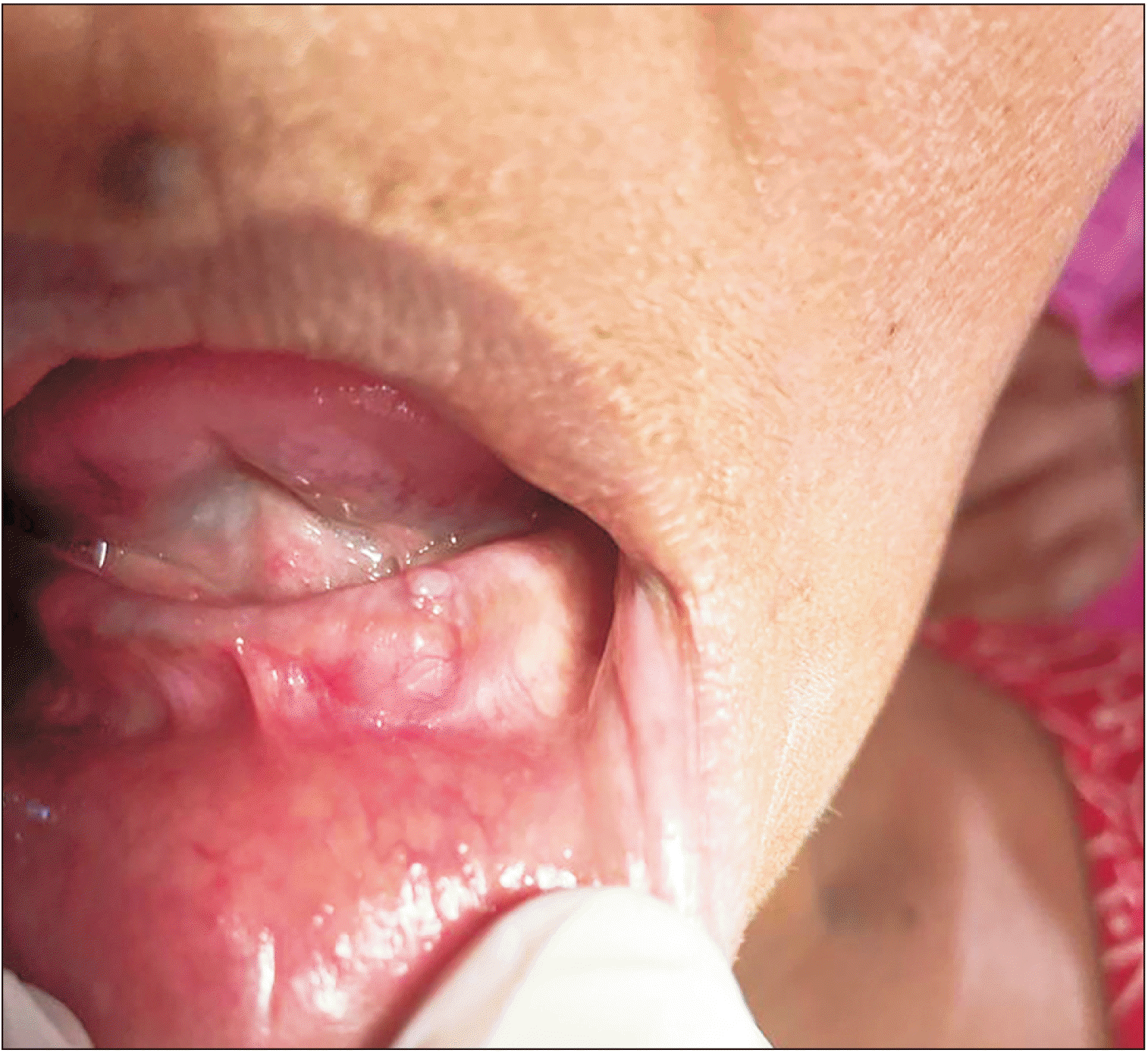
A 60-year-old female patient reported to the dental out patient department seeking a dental prosthesis. During an intra-oral examination, she was found to have a small dome-shaped swelling extending in the missing lateral incisor-canine region of the left mandibular alveolar ridge. The swelling was sessile, pale, non-tender, smooth in texture, firm in consistency, had well-defined margins, and was sitting on a normal-appearing mucosa. It measured 1.5 cm×1.3 cm (Fig. 1), did not tend to bleed, and blanched on palpation. The patient was asymptomatic and guessed that it had been there for approximately 6 years. She had no significant history of irritation/trauma, discharge/bleeding, previous ulceration, exposure to a relevant medication/drug, or hospitalization. Intraoral periapical radiograph and routine blood investigations were conducted and produced insignificant findings. A provisional diagnosis of fibroma with a differential diagnosis of peripheral giant cell granuloma was made. The tissue was sent for histopathological evaluation, and postoperative healing of the patient was uneventful.
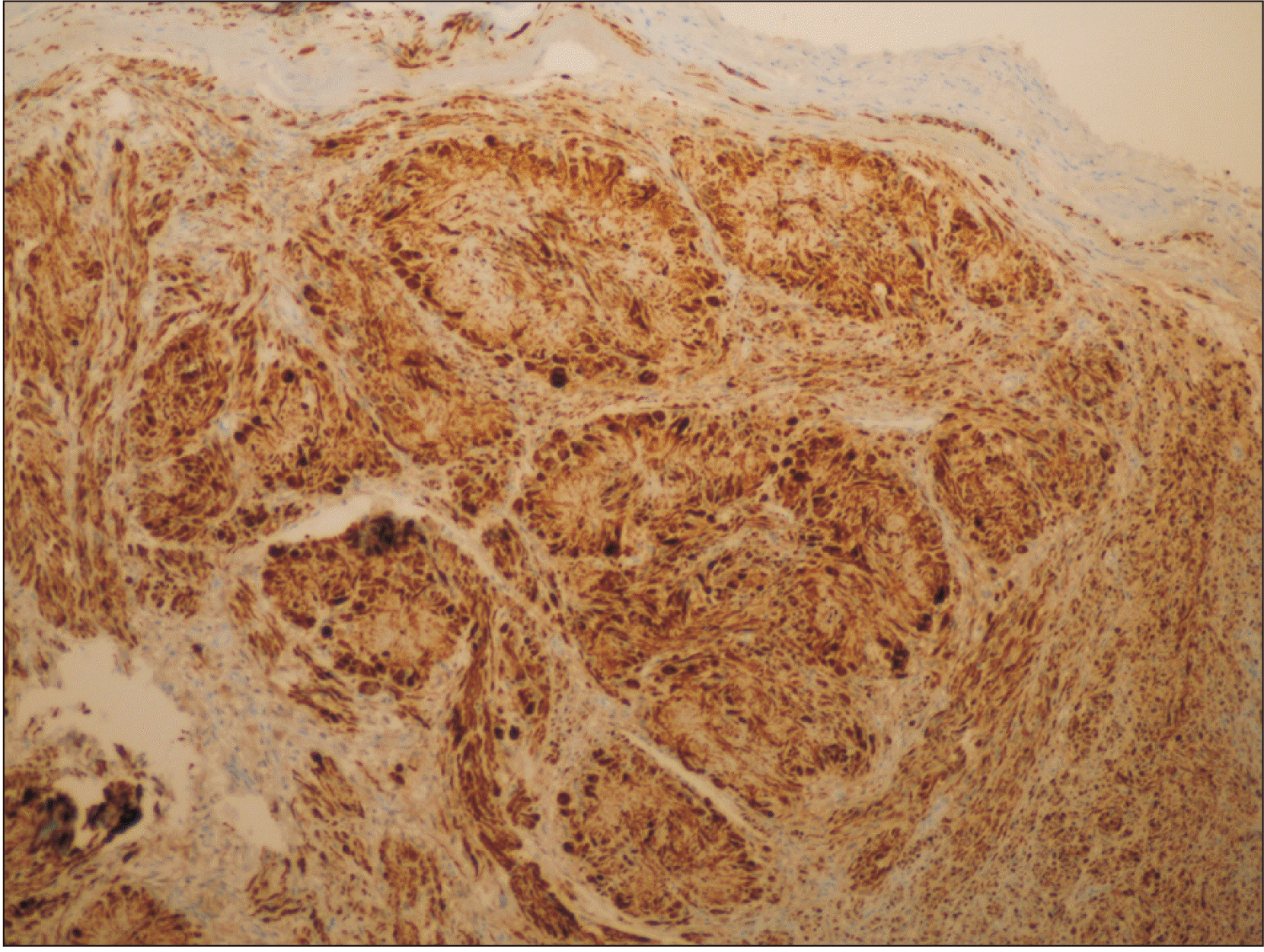
The sections showed a well-capsulated and bilocular lesion. The tumor was highly cellular with characteristic fibro-collagenous cores. The latter organization provided the pathology, a rosette or floret-like arrangement.(Fig. 2) The tumor cells around the irregularly-shaped rosettes were bland and polygonal, with ample cytoplasm giving it an epithelioid morphology with occasional binucleation/tri-nucleation, simulating a giant cell. The epithelioid cells showed a mild amount of cellular to nuclear pleomorphism without accompanying mitotic activity.(Fig. 3) A few long and thin spindle-shaped cells were seen entrapped in the variably sized collagen cores of the rosettes. The inter-rosette area was richly cellular and composed of spindle-shaped cells with buckled/crooked nuclei, resembling the Antoni A arrangement. Most of those cells resembled Schwann cells and showed long interlacing fascicular arrangements devoid of Verocay bodies. There was no evidence of Antoni B areas. Occasional blood vessels could be seen, a few engorged with red blood cells and others with thickened hyalinized periphery. The overlying epithelium showed evidence of pseudo-epitheliomatous hyperplasia, and the basilar cells exhibited a prominent increase in the amount of melanin pigment. Melanin incontinence and a few chronic inflammatory cells were seen dispersed on the densely fibrous stroma. No perineural or vascular invasion could be ascertained. Masson’s trichrome stain confirmed the deposition of blue collagen fibers arranged in a radiating pattern inside the rosette’s core.(Fig. 4)
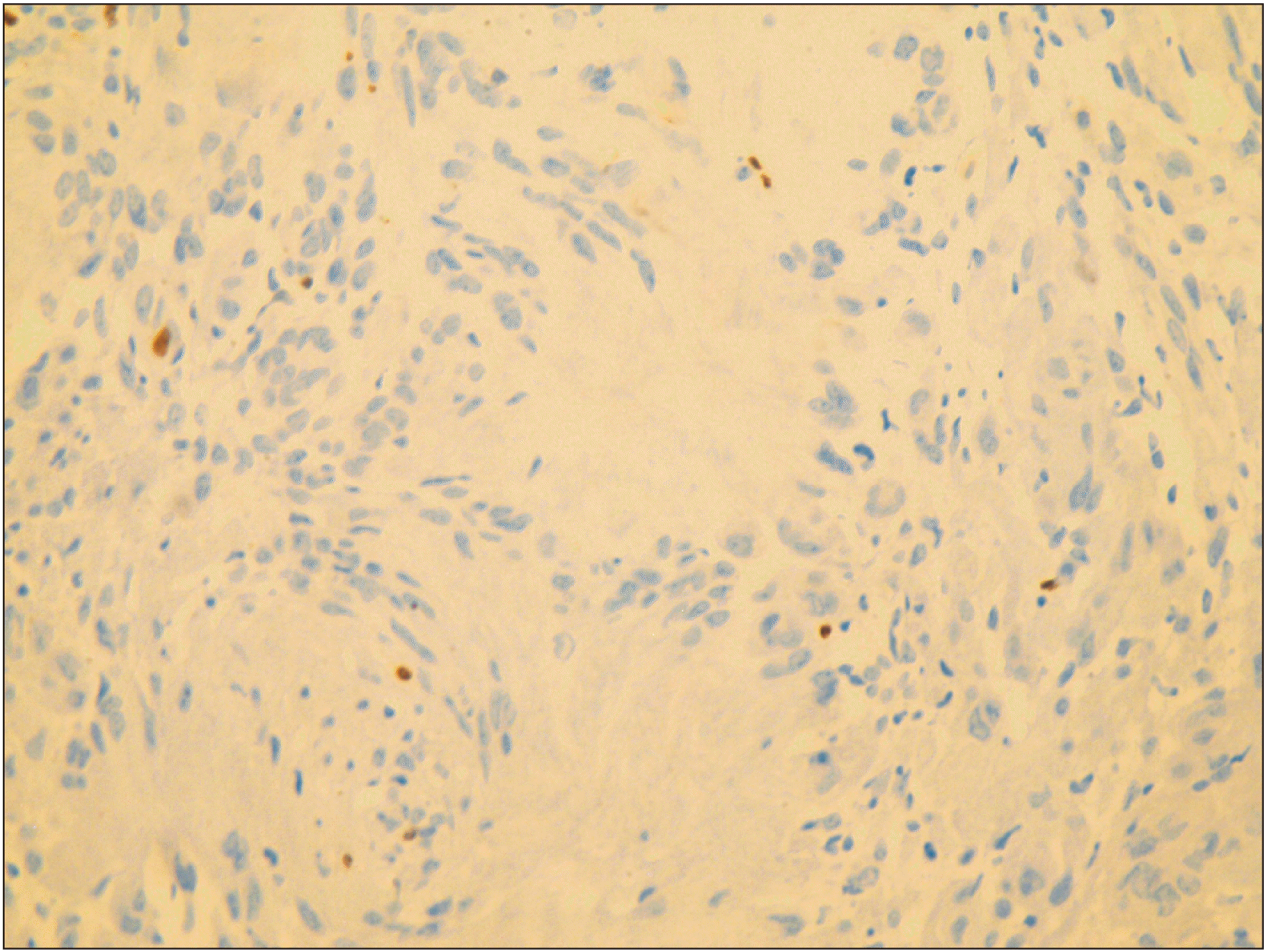
S-100 immunostain showed strong, diffuse deposition of nuclear and cytoplasmic brown, confirming the presence of a neural tumor of Schwann cell origin.(Fig. 5) Ki-67 immunomarker testing was performed to ascertain the proliferative status of the atypical cells seen, and the result was negative.(Fig. 6) Subsequently, a diagnosis of schwannoma with evidence of rosette and epithelioid change was made. The patient was followed up for 1 year and 2 months without any recurrence.
Schwannoma’s classical histopathological arrangement is called Antoni A and Antoni B and was first described by the Swedish neurologist Nils Antoni (1920). The palisading arrangement of Schwann cells in a stack-like arrangement with an acellular eosinophilic center is eponymically called Verocay bodies because they were first described by the Uruguayan neuropathologist Jose Verocay (1910)10. The anuclear zone of a typical Verocay body consists of the cytoplasmic processes from a stack of Schwann cells. Such an arrangement of nuclei occurs as an adaptive response to maintain cell–cell interactions that could be disrupted by increased matrix deposition of laminin and phospholipids such as lysophosphatidic acid in Schwann cells11.
Schwannomas originate histogenetically from the sheath of the peripheral nerves and present an array of histological features consistent with neural crest cells or neuroectodermal tissue, paving the way to diverse variants. Also, they present with a true capsule around them that is made up of epi- or perineurium instead of the condensed collagen fibers seen in other mesenchymal tumors12. The variants mentioned in the literature include ancient schwannoma (with degenerative changes, including marked nuclear atypia), cellular schwannoma (with high cellularity and the presence of mitotic activity), plexiform schwannoma (with multinodular/multiple discohesive patterns), epithelioid schwannoma (predominantly epithelioid changes), melanotic schwannoma (presence of melanin pigment and epithelioid change), glandular schwannoma (controversial glandular arrangements), Pacinian schwannoma (containing whorled structures), lymph node schwannoma (extremely rare; arises inside a lymph node), multiple schwannoma (schwannomatosis), and hybrid schwannoma/perineurioma (Antoni A areas with a whorled growth pattern)12-14. Other less commonly cited histopathological variants of schwannoma include neuroblastoma-like14 and microcystic variants15.
Our case displayed irregular rosettes/florets that resembled reported cases of neuroblastoma-like schwannomas13-15, but the cellular details were different. In neuroblastoma-like schwannomas, rosette-like arrangements of round, hyperchromatic Schwann cells were described by Goldblum et al.16 in 1994, and therefore primitive neuroectodermal tumors, Ewing’s sarcoma, neuroblastoma, and other neural crest pathologies became the differential diagnoses. Previously reported rosettes exhibited central collagenous cores13, and neuroblast-like cells have been described as hyperchromatic with indistinct nucleoli and a minimal amount of cytoplasm15,16. These tumors can present with minimal atypia as well. Since then, multiple cases have been reported in the literature, with the first case in the oral cavity described by Sedassari et al.17 in 2014. The present case showed rosette formations, but the cells around the rosette didn’t resemble the small, hyperchromatic, round cells of neuroblastoma; instead, they presented with ample eosinophilic cytoplasm and a bland nucleus. Thus, they resembled epithelioid cells rather than neuroblast cells. The epithelioid cells also showed mild cytological atypia without evidence of mitotic figures. The inter-rosette cells were spindle-shaped, resembling Schwann cells, with vesicular nuclei and prominent nucleoli. These cells made long fascicles, but no evidence of Verocay bodies could be seen. Vélez et al.14 used the descriptive term, rosetoid schwannoma to describe schwannomas with rosette patterns but no neuroblastoma-like features. Similar to Vélez et al.’s and Fisher et al.’s cases14,18, our oral variant showed rosette formations without neuroblastoma-like cells and with epithelioid differentiation. Therefore, we propose epithelioid schwannoma with rosettes as descriptive terminology for such cases13,18.
Epithelioid schwannomas are a rare variant of schwannoma described in the literature18,19. They contain no true epithelial components, that is, glandular or squamous differentiation18. Hart’s analysis of 58 cases of epithelioid schwannoma showed that most cases consisted predominantly of epithelioid cells, with variable amounts of stroma20. According to him, around one-third of the epithelioid areas gradually merged with the spindle-shaped cells, which occasionally displayed nuclear palisading. In 12% of the cases in Hart’s series, giant rosette-like collagenous deposition with a rim of epithelioid cells was seen, which was similar to our case. Dilated and hyalinized blood vessels were also found in most of his cases20. These epithelioid schwannomas are highly cellular and often do not show the features characteristic of Antoni A and B areas, which can mistakenly suggest malignancy. Considering the error in our pathology, we assessed the tumor for S-100 and Ki-67 to confirm the nature of the cells and rule out malignancy, respectively.
Ultra-structurally, Schwann cells show diffuse and strong nuclear and cytoplasmic staining with S-100, which is seen in our case. On the other hand, perineural cells and fibroblasts stain negative for S-100, which helps to distinguish non-neural pathologies and neural pathologies with mixed cellularity such as neurofibroma7. On the other hand, our tumor stained negative for Ki-67, which allowed us to rule out malignant behavior. Other markers commonly used for diagnosing schwannomas include the SOX-10 protein for intra-cellular, mature Schwann cells and collagen-IV and laminin for the pericellular/membranous component of Schwann cells. Epithelial membrane antigen can be used to show trapped perineurial components, often toward the periphery17.
Another interesting feature in our case is the bilobular pattern encircled by thin connective tissue septa. The literature shows a multilobular pattern in plexiform schwannomas, but a bilobular pattern with uncommon histopathology has not previously been mentioned, to the best of our knowledge.
Conventional schwannomas are generally encapsulated, and thus the prognosis is excellent after surgical extirpation, provided that any associated nerve is saved20. Malignant transformation has been seen in epithelioid/cellular and plexiform variants7. The presence of atypia in an epithelioid schwannoma doesn’t confirm a malignant transformation. On the other hand, malignant epithelioid schwannomas are frequently deep-seated and anaplastic20.
The superficial location of our asymptomatic, solitary pathology with a history of slow growth and long duration, along with well-demarcated borders, smooth glistening capsule, and easy enucleation, were all clinical signs of benignancy. However, histological evidence of rosettes, epithelioid cells, and mild atypia with a lack of mitotic activity and negative Ki-67 stain were required to definitively diagnose benignancy. S-100 positivity led to our definitive diagnosis of neurilemmoma with rosettes and epithelioid cells.
In conclusion, Schwannomas are rare and asymptomatic growths that originate from Schwann cells in the peripheral nervous system. The preoperative provisional diagnosis can be difficult to make, but their good encapsulation and smooth surgical appearance might orient clinicians toward that diagnosis. Conventional schwannomas can be easily diagnosed histopathologically, but in diagnosing its many variants, S-100 can help provide a definitive diagnosis. In rare variants with high cellularity, distinctive rosette formations, and epithelioid changes, a proliferative marker such as Ki-67 can help distinguish schwannomas from malignancy. Due to the rarity of this case, scant evidence is available about the nature and recurrence of such tumors.
Acknowledgements
We would like to thank Dr. Manisha Lakhanpal for providing clinical details related to the case.
Notes
Authors’ Contributions
M.M. wrote the manuscript. K.C. wrote the manuscript and reviewed the literature. V.K.S. read the proof and reviewed the manuscript. M.L. gave the clinical details of the case.
References
1. Heo SJ, Kim AY, Kim JS. 2022; Two cases of neurilemmoma in the nasal vestibule: a case report and literature review. Medicine (Baltimore). 101:e29006. https://doi.org/10.1097/md.0000000000029006. DOI: 10.1097/MD.0000000000029006. PMID: 35451397. PMCID: PMC8913084.

2. Urs AB, Kumar P, Augustine J, Malhotra R, Jot K. 2021; Pathological diversity in Schwannomas of the orofacial region. Asian J Neurosurg. 16:402–5. https://doi.org/10.4103/ajns.ajns_470_20. DOI: 10.4103/ajns.AJNS_470_20. PMID: 34268175. PMCID: PMC8244711.

3. Sitenga J, Aird G, Vaudreuil A, Huerter CJ. 2018; Clinical features and management of Schwannoma affecting the upper and lower lips. Int J Dermatol. 57:1047–52. https://doi.org/10.1111/ijd.13920. DOI: 10.1111/ijd.13920. PMID: 29377087.

4. Bakir M, Enabi J, Almeshal H. 2021; Unusual lower lip swelling: a rare case of lip Schwannoma. Cureus. 13:e19242. https://doi.org/10.7759/cureus.19242. DOI: 10.7759/cureus.19242. PMID: 34900449. PMCID: PMC8647776.

5. Lee JD, Kwon TJ, Kim UK, Lee WS. 2012; Genetic and epigenetic alterations of the NF2 gene in sporadic vestibular Schwannomas. PLoS One. 7:e30418. https://doi.org/10.1371/journal.pone.0030418. DOI: 10.1371/journal.pone.0030418. PMID: 22295085. PMCID: PMC3266248.

6. Cardoso VS, Quelemes PV, Amorin A, Primo FL, Gobo GG, Tedesco AC, et al. 2014; Collagen-based silver nanoparticles for biological applications: synthesis and characterization. J Nanobiotechnology. 12:36. https://doi.org/10.1186/s12951-014-0036-6. DOI: 10.1186/s12951-014-0036-6. PMID: 25223611. PMCID: PMC4428528.

7. Nishijima Sakanashi E, Sonobe J, Chin M, Bessho K. 2015; Schwannoma located in the upper gingival mucosa: case report and literature review. J Maxillofac Oral Surg. 14(Suppl 1):222–5. https://doi.org/10.1007/s12663-012-0445-8. DOI: 10.1007/s12663-012-0445-8. PMID: 25838700. PMCID: PMC4379268.

8. Belakhoua SM, Rodriguez FJ. 2021; Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 88:443–56. https://doi.org/10.1093/neuros/nyab021. DOI: 10.1093/neuros/nyab021. PMID: 33588442. PMCID: PMC7884141.

9. Qi Z, Yang N, Pi M, Yu W. 2021; Current status of the diagnosis and treatment of gastrointestinal Schwannoma. Oncol Lett. 21:384. https://doi.org/10.3892/ol.2021.12645. DOI: 10.3892/ol.2021.12645. PMID: 33777207. PMCID: PMC7988712.

10. Joshi R. 2012; Learning from eponyms: Jose Verocay and Verocay bodies, Antoni A and B areas, Nils Antoni and Schwannomas. Indian Dermatol Online J. 3:215–9. https://doi.org/10.4103/2229-5178.101826. DOI: 10.4103/2229-5178.101826. PMCID: PMC3505436. PMID: 23189261.

11. Weiner JA, Fukushima N, Contos JJ, Scherer SS, Chun J. 2001; Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J Neurosci. 21:7069–78. https://doi.org/10.1523/jneurosci.21-18-07069.2001. DOI: 10.1523/JNEUROSCI.21-18-07069.2001. PMID: 11549717. PMCID: PMC6763011.

12. Weiss SW, Goldblum JR. 2008. Enzinger and Weiss's soft tissue tumors. 5th ed. Elsevier.
13. Koubaa Mahjoub W, Jouini R, Khanchel F, Ben Brahim E, Llamas-Velasco M, Helel I, et al. 2019; Neuroblastoma-like Schwannoma with giant rosette: a potential diagnostic pitfall for hyalinizing spindle cell tumor. J Cutan Pathol. 46:234–7. https://doi.org/10.1111/cup.13405. DOI: 10.1111/cup.13405. PMID: 30582192.

14. Vélez D, Reina Duran T, Pérez-Gala S, Fernández JF. 2006; Rosetoid Schwannoma (neuroblastoma-like) in association with an anetoderma. J Cutan Pathol. 33:573–6. https://doi.org/10.1111/j.1600-0560.2006.00480.x. DOI: 10.1111/j.1600-0560.2006.00480.x. PMID: 16919032.

15. Suchak R, Luzar B, Bacchi CE, Maguire B, Calonje E. 2010; Cutaneous neuroblastoma-like schwannoma: a report of two cases, one with a plexiform pattern, and a review of the literature. J Cutan Pathol. 37:997–1001. https://doi.org/10.1111/j.1600-0560.2009.01455.x. DOI: 10.1111/j.1600-0560.2009.01455.x. PMID: 19922484.

16. Goldblum JR, Beals TF, Weiss SW. 1994; Neuroblastoma-like neurilemoma. Am J Surg Pathol. 18:266–73. https://doi.org/10.1097/00000478-199403000-00006. DOI: 10.1097/00000478-199403000-00006. PMID: 8116794.

17. Sedassari BT, da Silva Lascane NA, Cury Gallottini MH, Orsini Machado de Sousa SC, Pinto Júnior Ddos S. 2014; Neuroblastoma-like Schwannoma of the lower labial mucosa: a rare morphologic variant of peripheral nerve sheath tumor. Oral Surg Oral Med Oral Pathol Oral Radiol. 118:579–82. https://doi.org/10.1016/j.oooo.2014.05.023. DOI: 10.1016/j.oooo.2014.05.023. PMID: 25174314.

18. Fisher C, Chappell ME, Weiss SW. 1995; Neuroblastoma-like epithelioid Schwannoma. Histopathology. 26:193–4. https://doi.org/10.1111/j.1365-2559.1995.tb00654.x. DOI: 10.1111/j.1365-2559.1995.tb00654.x. PMID: 7737668.

19. Shim SK, Myoung H. 2016; Neurilemmoma in the floor of the mouth: a case report. J Korean Assoc Oral Maxillofac Surg. 42:60–4. https://doi.org/10.5125/jkaoms.2016.42.1.60. DOI: 10.5125/jkaoms.2016.42.1.60. PMID: 26904498. PMCID: PMC4761576.

20. Hart J, Gardner JM, Edgar M, Weiss SW. 2016; Epithelioid Schwannomas: an analysis of 58 cases including atypical variants. Am J Surg Pathol. 40:704–13. https://doi.org/10.1097/pas.0000000000000589. DOI: 10.1097/PAS.0000000000000589. PMID: 26752543.

Fig. 2
Multiple rosette-like arrangements of tumor cells with fibro-collagenous cores surrounded by fascicles of spindle-shaped tumor cells (H&E staining, ×10).
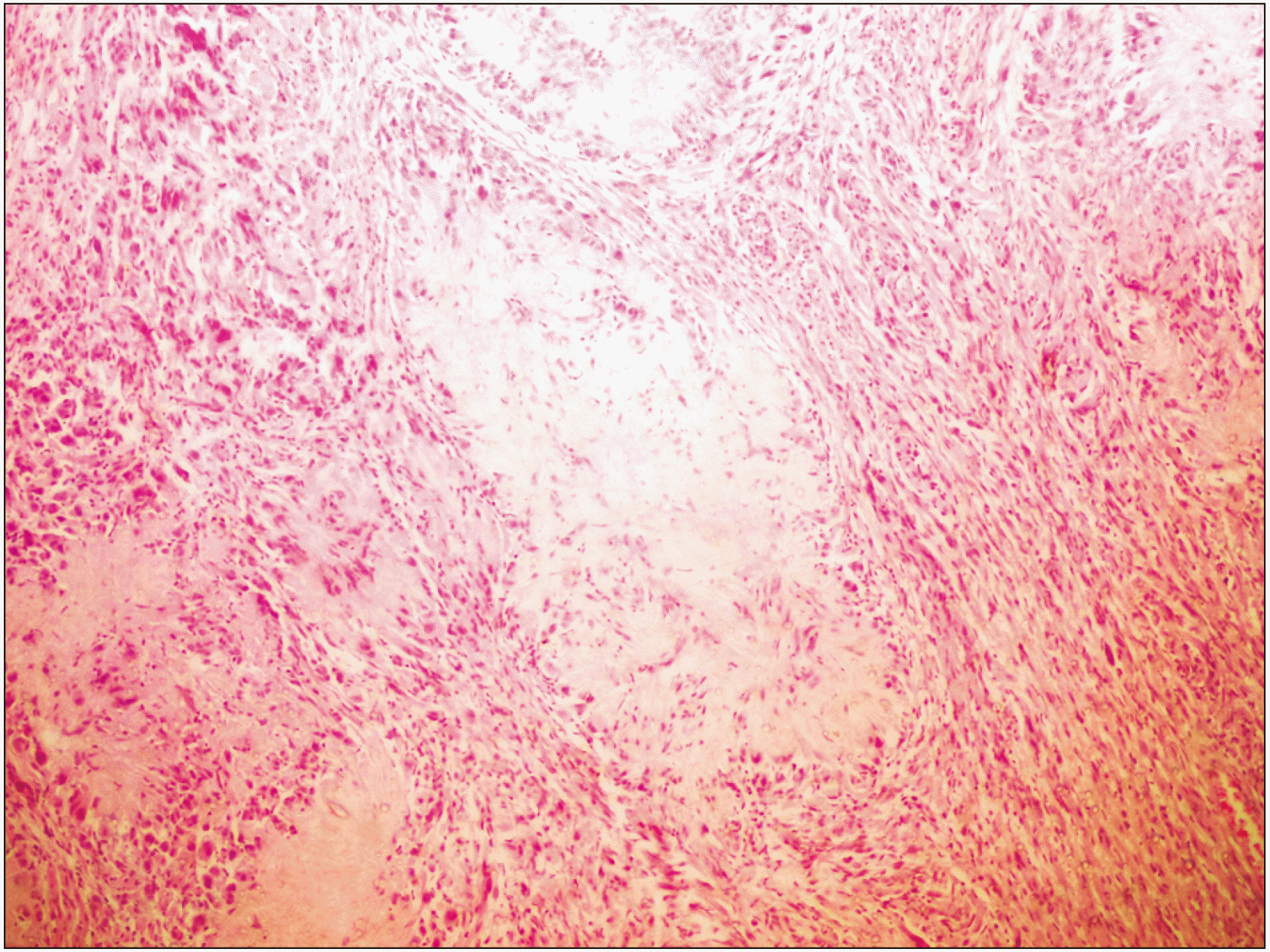
Fig. 3
A single rosette with epithelioid cells encircling the rosette-like radiating fibro-collagenous cores with mild amount of atypia. The tumor cells on the left side are spindle-shaped resembling typical Schwann cells (H&E staining, ×40).
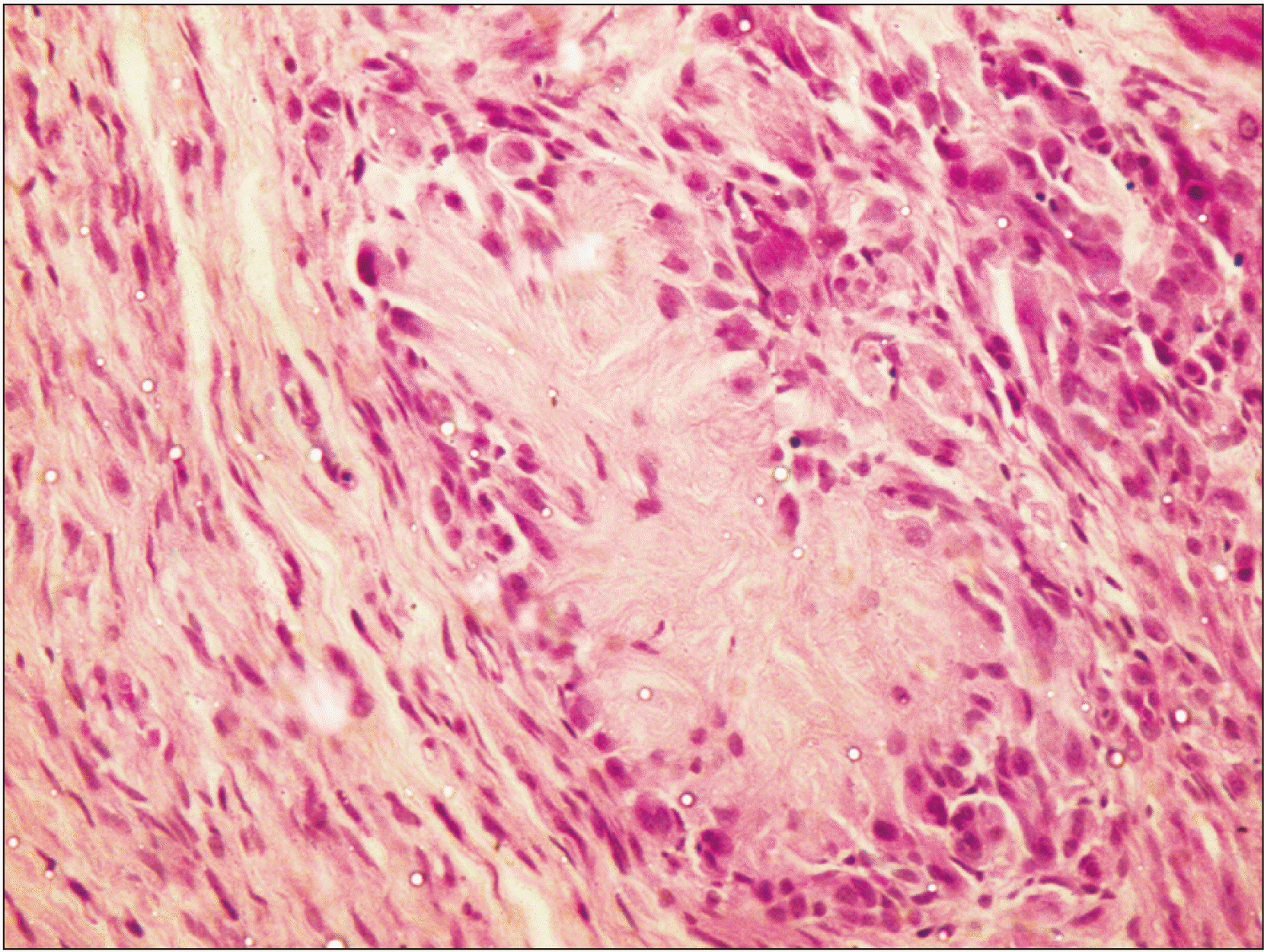
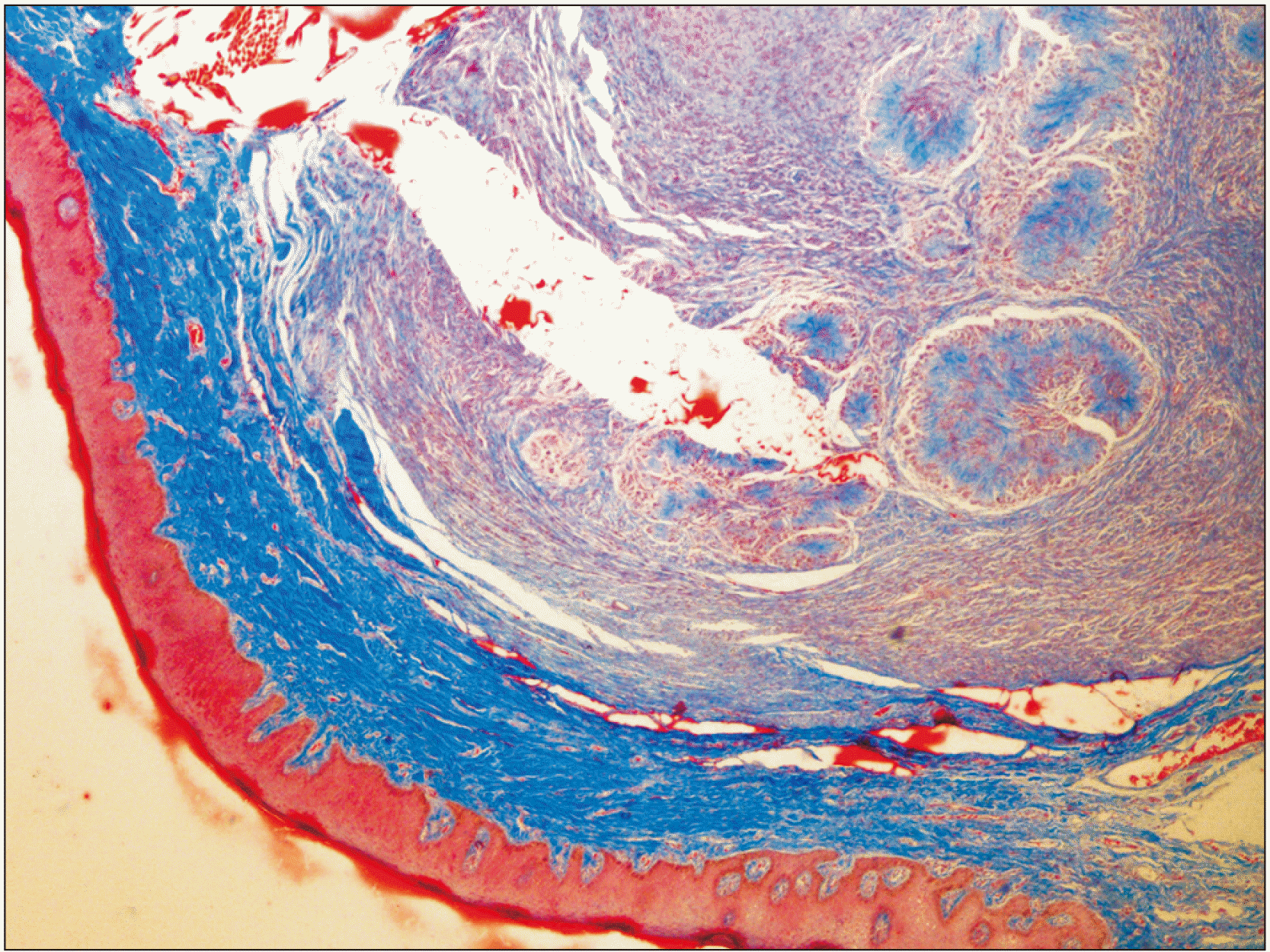
Fig. 4
The blue-colored center of rosettes and the periphery of the tumor confirm the presence of collagen fibers (MT staining, ×4).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download