Case
Ethical statements: This study was approved by the Ethics Committee of the Pusan National University Hospital (approval no. 2022-0392). The study participant was provided with informed written consent prior to treatment and study enrollment.
A 27-year-old man (height, 168 cm; body weight, 90 kg; body mass index, 31.89 kg/m2) underwent elective surgery for metal removal in the left humerus and ulna. He was admitted with multiple tendon injuries, left humerus shaft fracture, left ulnar shaft fracture, and multiple lacerations after an industrial accident 3 years ago. He had a history of four surgeries, including open reduction and internal fixation twice, closed reduction and internal fixation once, and tendon transfer once, under BPB with light sedation using dexmedetomidine, from August 2018 to March 2019. No abnormal findings were observed in the electrocardiogram, chest X-ray, or laboratory parameters.
In the operating room, electrocardiogram, noninvasive blood pressure (NIBP) measurement, and pulse oximetry were monitored. A cuff for NIBP measurement was placed on the right arm, and a pulse oximeter was attached to the patient's right big toe to detect saturation by pulse oxymetry (SpO2). Ten minutes after BPB under ultrasound guidance, sensory and motor tests were performed at the site to be operated. After confirming adequate anesthesia, the surgeon was informed to start the operation. The patient was notified of sedative use and dexmedetomidine was loaded at 1.0 μg/kg for 10 minutes when the surgical area was cleaned with an antiseptic solution and draped with sterile sheets; the patient was maintained on light sedation, and the SpO2 was 95%. Therefore, we supplied oxygen to the patient at 3 L/min through nasal prong and continued intravenous infusion by changing the dexmedetomidine to a maintenance dose of 0.5 μg/kg/hr, and the patient maintained the light sedation state with SpO2 at 99%.
After 30 minutes of BPB, a skin incision was made in the distal ulna for surgery, and the patient complained of pain at the incision site. After consulting with the operating surgeon, it was determined that anesthesia through BPB was incomplete. Following, subcutaneous injection of 1% lidocaine into the skin incision, it was decided to proceed with the operation. Thereafter, the operation was performed for 10 minutes, but there was no motion or pain complaint in the patient. The patient complained of pain again when manipulating the distal ulna to remove the metal, but the intensity was mild and there was no movement. The surgeon and anesthesiologist assessed that the patient's pain was minor and the surgery was decided to be continued by controlling the pain. Thus, continuous injection of remifentanil to the patient at 0.05 μg/kg/min was started. The operation was performed without any complaints of pain, and the patient's vital signs were as follows: heart rate (HR), 77 beats per minute (bpm); NIBP, 126/85 mmHg; SpO
2, 95%. The intravenous infusion of remifentanil was reduced to 0.01 μg/kg/min, and the continuous infusion of dexmedetomidine for sedation was continued at 0.1 μg/kg/hr (
Fig. 1).
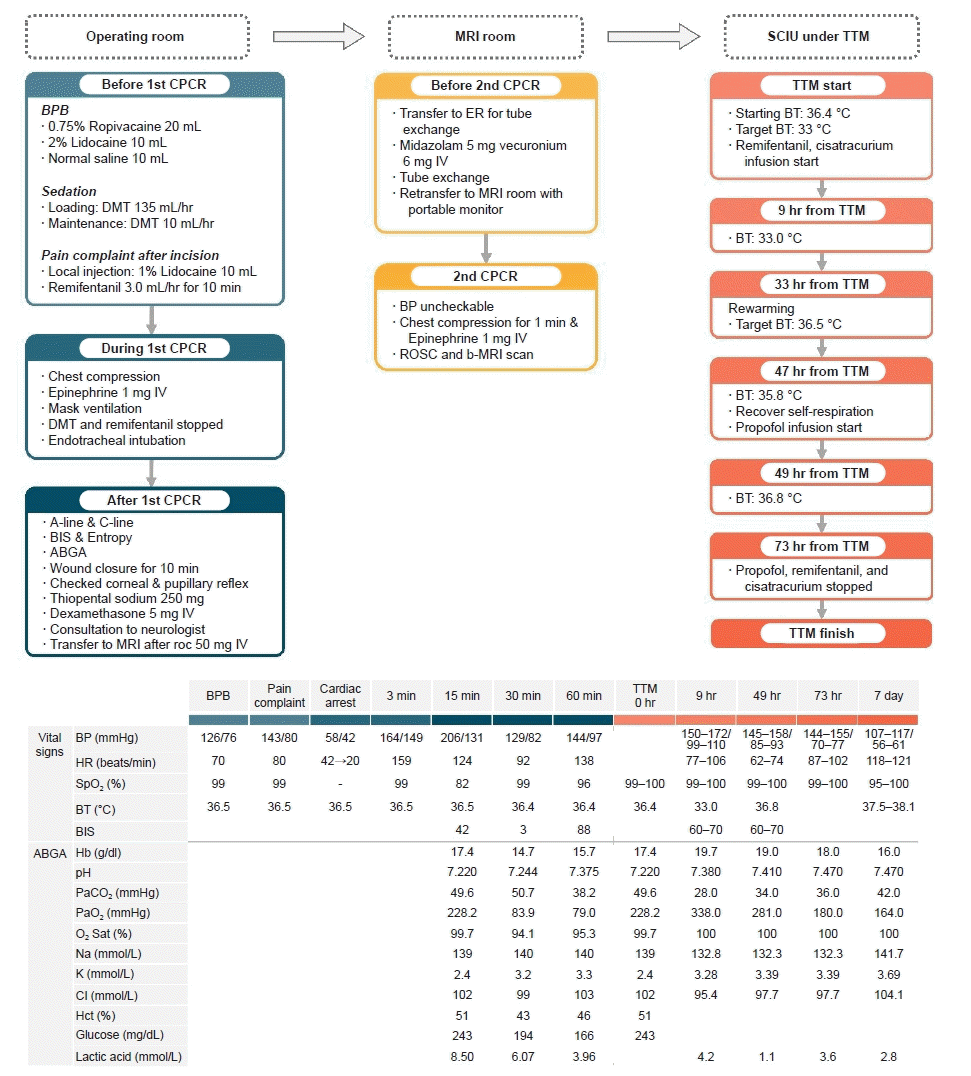 | Fig. 1.Timeline of cardiopulmonary resuscitation and patient treatment with changes in vital signs, ABGA, electrolytes, glucose, and lactic acid. MRI, magnetic resonance imaging; SCIU, surgical intensive care unit; TTM, targeted temperature management; CPCR, cardiopulmonary cerebral resuscitation; BPB, brachial plexus block; DMT, dexmedetomidine; IV, intravenous; BIS, bispectral index; ABGA, arterial blood gas analysis; ER, emergency room; BP, blood pressure; ROSC, return of spontaneous circulation; b-MRI, brain MRI; BT, body temperature; HR, heart rate; SpO2, saturation by pulse oximetry; Hb, hemoglobin; PaCO2, arterial carbon dioxide pressure; O2 Sat, SaO2 saturation of arterial oxygen; PaO2, arterial oxygen pressure; Hct, hematocrit. 
|
A pulse oximeter was attached to the patient's right big toe for SpO
2 detection. At that time the patient's SpO
2 was not detected well, so we then moved the sensor to the patient's left big toe. After replacing the SpO
2 measuring cable and module, when the anesthesiologist approached the patient's head, sudden bradycardia (HR, 42 bpm) and hypotension (NIBP, 58/45 mmHg) were observed with a monitor alarm. The tenting was rolled up to check the patient's condition. Cyanosis and apnea were observed. The surgeon was instructed to stop the operation immediately and we continued waking up the patient to restore his consciousness. While connecting the mask to secure the airway, the patient's HR decreased to 20 bpm. Chest compression was immediately performed, and 1 mg of epinephrine was simultaneously administered intravenously. Intravenous infusion of dexmedetomidine and remifentanil was immediately stopped, tracheal intubation was performed by another anesthesiologist 2 minutes after the start of chest compression. Return of spontaneous circulation (ROSC) was observed 3 minutes after the beginning of chest compression and the patient’s vital signs were as follows: NIBP, 164/149 mmHg; HR, 159 bpm; and SpO
2, 99% (
Fig. 1).
Assuming that the pulse oximeter was not detected at the onset of respiratory failure, the total time for which oxygen had not been supplied to the patient was estimated to be up to 7 minutes.
Arterial blood pressure (ABP) and central venous pressure were monitored immediately via the right radial artery and the right subclavian vein. The bispectral index (BIS; BIS VISTA, Aspect Medical Systems, Inc., Norwood, MA, USA) sensor and spectral entropy (Entropy sensor, Datex-Ohmeda, Helsinki, Finland) were simultaneously attached to his forehead to measure his awareness. Fifteen minutes after CA, the patient's vital signs were as follows: ABP, 206/131 mmHg; HR, 159 bpm; SpO
2, 82%; and end-tidal CO
2, 52 mmHg (
Fig. 1). The results of arterial blood gas analysis (ABGA) are shown in
Fig. 1. The metal had already been removed from the left distal ulna, but the wound was still open; therefore, the wound was closed over the next 10 minutes without any sedative or opioids before leaving the operating room. At that time, the entropy was 52, and the BIS was 42 (
Fig. 1). The metals in the humerus were not removed, but we decided to stop the operation.
We continued the effort to restore the patient's consciousness 30 minutes after the resuscitation; however, the patient's entropy gradually decreased to 5 and the BIS score was 3, and the pupillary and corneal reflexes were still lost on both sides. The patient did not recover consciousness, could not use facial muscles, and could not obey any command even though he recovered self-respiration. The results of the ABGA done at that time are shown in
Fig. 1.
The patient's entropy and BIS gradually increased to 100 and 88, respectively, but the pupillary and corneal reflexes could not be observed in both eyes at 60 minutes after resuscitation. We suspected HIBI because all reflexes and movements of the patient were restricted despite the increase in BIS and entropy. Thiopental sodium (250 mg) and dexamethasone (5 mg) were administered via the central vein for brain protection from HIBI. Following the advice of a neurologist, after contacting the patient's family members to explain the situation and obtain consent over the cellular phone, it was decided to perform brain magnetic resonance imaging (b-MRI). Rocuronium (50 mg) was administered to the patient to confirm the cessation of spontaneous breathing, followed by Ambu bagging, and the patient was transferred to the MRI room.
Before starting the b-MRI scan, it was confirmed that the endotracheal tube was a reinforced tube unsuitable for the scan. The patient was transferred to the nearest emergency room, a plain endotracheal tube was changed and re-transferred to the MRI room. A portable ventilator was applied to the patient, and the ABP, HR, and SpO2 were monitored. When the medical staff left the MRI room at the onset of the imaging, the patient's ABP and HR were measured as 50/23 mmHg and 30 bpm, respectively. Chest compression was immediately performed, and 1 mg of epinephrine was intravenously injected through the central vein. The patient was resuscitated and subsequently, the patient's ABP, HR, and SpO2 were 180/110 mmHg, 123 bpm, and 95%, respectively. The b-MRI was successfully performed under close monitoring and reported as "No evidence of acute infarction."
The patient was transferred to the surgical intensive care unit and we decided to perform TTM following the advice of a neurologist due to the possibility of HIBI. The patient’s pupillary and corneal reflexes could not be observed. Remifentanil and cisatracurium were continuously administered, and TTM was applied with the target BT at 33 °C. The BT at the beginning of TTM was 36.4 °C, and the BT reached 33 °C at 9 hours after the start of TTM. The vital signs and ABGA results are shown in
Fig 1.
After applying TTM for 24 hours (33 hours from TTM initiation), rewarming was initiated by inducing an increase in BT. At 14 hours after the start of the ascent (47 hours from TTM initiation), the patient began to recover with a little spontaneous respiration, and the BIS was maintained between 70 and 80. Remifentanil and cisatracurium infusion were checked again, and propofol infusion was started continuously. During the 16 hours from the start of the ascent of BT (49 hours from TTM initiation), the BT increased by 0.25 °C per hour and reached 36.8 °C. The vital signs and ABGA results of this duration are shown in
Fig. 1.
The patient was maintained at normal BT for 24 hours (73 hours from TTM initiation), and there were no abnormal findings during that time. Subsequently, the application of TTM and the continuous intravenous infusion of propofol, remifentanil, and cisatracurium, which were administered to the patient, was stopped. Twenty minutes after the medication was discontinued, the patient maintained stable spontaneous breathing and was able to communicate with the medical staff; therefore, ventilator weaning was performed. The patient's vital signs measured at the time of weaning were stable: HR, 87–102 bpm; ABP, 144–154/70–77 mmHg; and SpO
2, 99%–100%. As shown in
Fig. 1, the ABGA results and laboratory findings were not unusual. Extubation was performed after confirming that the patient's consciousness was restored and that the muscle power was recovered to facilitate spontaneous breathing and supplied 5.0 L/min of oxygen to the patient using a reserve mask bag. After extubation, the patient complained of slight muscle weakness but was conscious and cooperative. The vital signs were stable, and laboratory tests did not reveal any specific findings. Interviews with guardians were conducted, and speech was poor, but communication was possible without major problems. Haloperidol was administered because of slight delirium, and there were no specific findings on b-MRI. The electroencephalogram (EEG) findings at that time were "suggestive of mild diffuse cerebral dysfunction" (
Fig. 2).
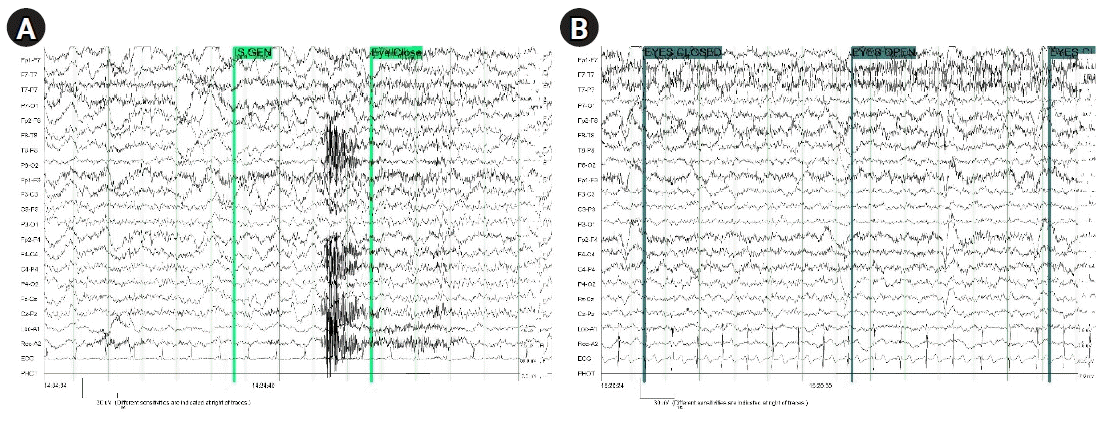 | Fig. 2.Electroencephalography findings were suggestive of mild diffuse cerebral dysfunction. (A) During target temperature management. (B) After regaining of consciousness. 
|
On the 7th day after the start of TTM, the patient was transferred to a general ward. On the 10th day after the start of TTM, the Korean-Mini Mental Status Examination test was normal with 27 points (normal, ≥25). On the 14th day after the start of TTM, the patient showed tandem gait and ataxia. Gait therapy was initiated at the rehabilitation medicine department. On the 16th day after surgery, the patient had normal somatosensory evoked potentials and motor evoked potentials. On the 28th day after the surgery, the patient complained of difficulty in prolonged one-leg standing and leg trembling; however, on the 33rd day after the surgery, the patient was discharged. He is currently undergoing outpatient treatment and has recovered without any sequelae.
Go to :

Discussion
Dexmedetomidine is a sedative with modest analgesic effect and remifentanil is an opioid analgesic with modest sedative effect [
3]. Remifentanil and dexmedetomidine alone, as well as combined administration, may cause side effects such as suppressing the patient's respiration or causing severe bradycardia [
3-
5]. Therefore, close monitoring is essential, especially when used as a combination. Cardiopulmonary collapse can result in neurological sequelae or sudden death after successful recovery due to HIBI and/or reperfusion brain injury [
6]. The BIS value commonly approached to zero following CA, and remained low despite successful cardiopulmonary cerebral resuscitation (CPCR) due to remnant anesthetics [
7]. The BIS value based on quantitative EEG may be associated with the prognosis of CA after CPCR [
6].
We have recognized that some questions may be raised as follows. First, it is unclear whether HIBI has occurred in successfully resuscitated patients or not. We predicted the possibility of HIBI occurrence in patients by closely observing the patient's absence of both corneal and pupillary reflex and patient’s posture in the form of decerebrate rigidity, as well as using the means to determine the arousal level such as BIS and entropy. In addition, three skillful anesthesiologists continued to try to wake the patient for a longer period of time than the expected time to normal awakening, but the patient's response was not observed, so the possibility of HIBI incidence was predicted. The reason for judging the patient's condition without going through immediate EEG or other additional work-up was to avoid deteriorating the patient's condition by delaying time for accurate diagnosis or to miss the golden time. From a risk-benefit point of view, even without direct evidence of HIBI in the patient, it was deemed necessary for the administration of procedures to help the patient recover normally by not delaying time of judgment. As a result, the patient resuscitated successfully and was discharged with minimal sequelae. Therefore, we are confident that these questions will not diminish the value of our case report and discussions.
Second, it is questionable how much the application of TTM played a role in the successful resuscitation of patients. In fact, no significant changes were observed in the patient's brain immediately after resuscitation by perfusion b-MRI. Severe HIBI can be diagnosed mainly through the absence of both corneal and pupillary reflexes, short delayed somatosensory-induced N
2O waves, high blood-specific enolases, adverse patterns of brain electrons, and brain computed tomography or MRI images [
8]. However, there is no only one mean to immediately and accurately determine whether HIBI has occurred. Perfusion b-MRI is not a suitable tool for diagnosing HIBI in the acute phase but is useful for diagnosing it between day 1 and day 5 after ROSC [
8]. Moreover, even BIS, entropy, and EEG cannot detect the occurrence of HIBI immediately and accurately [
9]. Somatosensory evoked potential amplitudes are not a suitable tool for diagnosing HIBI in the acute phases but are useful for diagnosing it between day 1 and day 4 after ROSC [
9]. So, we made such a judgment clinically, and the basis for this judgment was comprehensive as mentioned above. Once that judgment has been made, the procedure we can do is quite limited. we applied the TTM to the patient immediately. Although controversial, we also applied the “normobaric-hyperoxia” to the patient. It is not known exactly what was the decisive measure for suppressing the occurrence of HIBI in patients, but we believe that appropriate treatment was administered. The therapeutic mechanism of normobaric-hyperoxia increases the oxygen content of brain tissue and increases the function of mitochondria, enhancing the metabolism of neurons and reducing damage to the blood-brain barrier [
10]. In addition, brain cell edema is reduced and brain blood flow is reduced, resulting in brain pressure reduction and secondary brain blood flow improvement [
10]. It is to make neurons alive, especially in penumbral area, around injured areas of the brain [
10].
There were some parts of the treatment that were mishandled. First, performed incomplete partial anesthesia, and were unable to detect the problem through physical examination. The patient was scheduled for incision in two locations, ulnar and humerus, and there is always the possibility of inappropriate regional anesthesia even if BPB through supraclavicular approach was appropriate at each site in the previous three surgeries. In addition to overlooking this, if incomplete anesthesia was found quickly through a more detailed physical examination after anesthesia, retry of partial anesthesia or additional partial anesthesia using other approaches would have been performed. If even that was not enough, we had to change to general anesthesia.
Second, if the patient's SpO2 was not properly measured through the pulse oximeter, physical examination such as observation of the patient's breathing status or skin color should be preceded and vital signs should have been measured. However, by first recognizing it as a problem with the instruments and implementing the next best measures, such as replacing the pulse oximeter and cable, the patient missed the chance of spontaneous respiration, which was decisive for the patient. This misjudgment resulted in several complicated follow-up observations, and not only the patient and family but also the medical staff suffered a lot of time and money for recovery.
Third, chest compression was performed in the process of propoer resuscitation, the preparation of the corrugating tube to the anesthesia station and the Ambu bag was delayed. Due to the nature of local anesthesia with additional sedative usage, the means to secure airway should have been ready-to-use state, but there were time delay to prepare proper equipment.
In addition, because we urgently performed tracheal intubation by using the reinforced tube which was inappropriate for MRI scans, the tracheal tube had to be exchanged. Although the 2020 Advanced Cardiovascular Life Support guideline mentions that the priority application of chest compression is important, it is recommended that an appropriate oxygen mask or tracheal intubation to be applied during CPCR by experts. It can be said that it is another mistake.
Key aspects after ROSC, in addition to TTM, include appropriate ventilator management, hemodynamic optimization, identifying and addressing precipitating pathologic condition, and prognostication [
11].
To summarize based on our case: (1) It is important to remember that when performing the regional anesthesia it can always be incomplete, and before injecting sedatives, you should allow enough time to accurately determine whether adequate anesthesia for surgery has been performed; (2) When incomplete regional anesthesia is confirmed, it is necessary to quickly switch to monitored anesthesia care or general anesthesia to secure appropriate monitoring and airway; (3) When performing regional anesthesia with sedatives, appropriate tools should always be prepared to secure the necessary airway. In addition, if tracheal intubation is performed using an appropriate tube in the first place when applying CPCR, it may be a means to avoid the discomfort and risk of tube exchange that may occur; (4) Combination of remifentanil infusion during sedation using dexmedetomidine can cause severe apnea in obese patient. Therefore, it is recommended to avoid using it together for the purpose of sedation and analgesia effect during incomplete regional anesthesia; (5) If HIBI is suspected in a resuscitated patient after CPCR, the active and immediate application of TTM should be sufficiently considered to minimize the patient's brain damage [
1,
12]; (6) If TTM is applied, it is important to do it correctly as recommended for a sufficient time. Whether spontaneous breathing or recovery of consciousness of the patient does not occur during the period of BT rise should be determined through closed monitoring, and active measures to prevent or suppress this should be taken.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download