INTRODUCTION
MATERIALS AND METHODS
Study design
PM exposure model
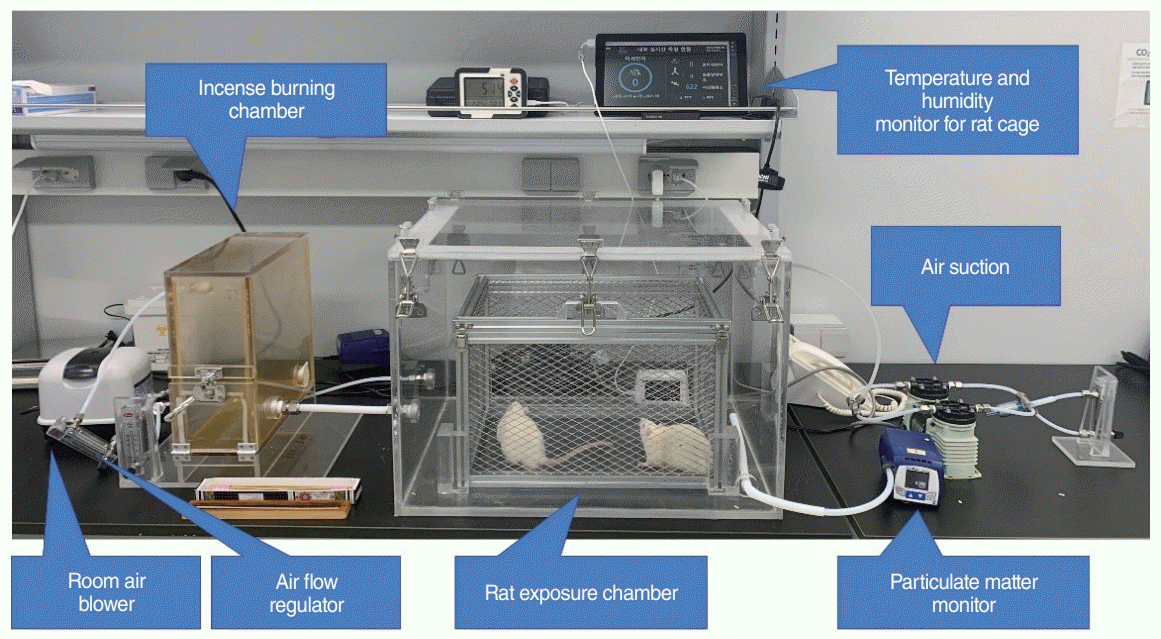 | Fig. 2.Particulate matter exposure model for rats. The blower supplies room air to the incense burning and rat exposure chambers. The incense smoke from the incense burning chamber is supplied to the rat exposure chamber, and the internal environment is monitored using the particulate matter (PM) monitor and temperature and humidity monitor. The amount of PM is controlled by the airflow regulator. Air suction is installed in the rat exposure chamber for continuous ventilation. |
Histopathological analysis
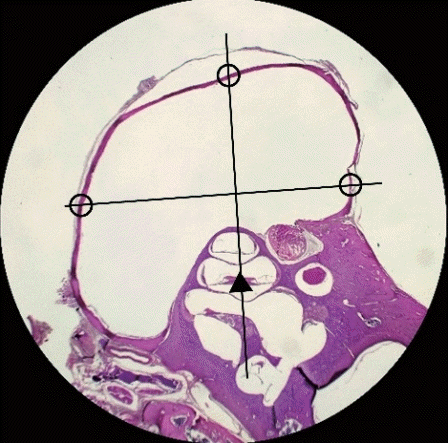 | Fig. 3.Measurement of the middle ear mucosa thickness in the control group under light microscopy (H&E, ×12.5). The average thickness of the middle ear mucosa was calculated by measuring the thickness at three sites (black circles): one at the mucosa on the contralateral side of the cochlea (black triangle), and the other two at the bilateral mucosa perpendicular to the imaginary line between the cochlea and the contralateral mucosa. |
Analysis of middle ear mucosa using RT-PCR
Table 1.
Statistical analyses
RESULTS
Histopathologic analysis of the ET and middle ear mucosa
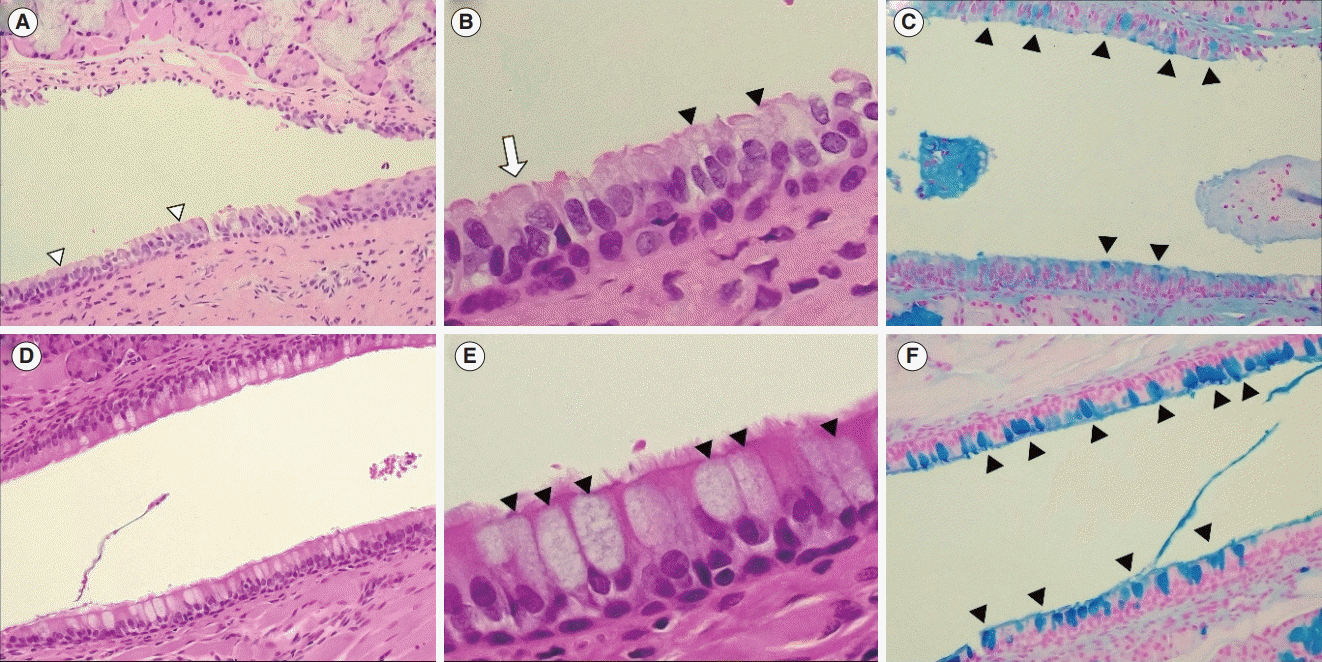 | Fig. 4.Histopathologic features of the Eustachian tube (ET) mucosa. (A) Light microscope findings of the ET in the control group (H&E, ×100). The pseudostratified mucociliary respiratory-like epithelium is observed (arrowheads). (B) High magnification image of ET mucosa in the control group (H&E, ×400). Cilia of the epithelial cell (arrow) and goblet cells (arrowheads) are shown on the ET mucosa. (C) Goblet cells (arrowheads) in the ET mucosa are observed in the control group (Alcian blue stain, ×100). (D) Light microscope findings of the ET in the 3-day exposure group (H&E, ×100). (E) High magnification image of ET mucosa in the 3-day exposure group (H&E, ×400). An increased number of
hypertrophied goblet cells (arrowheads) is shown. (F) An increased number of goblet cells (arrowheads) of ET mucosa is observed in the 3-day exposure group (Alcian blue stain, ×100). |
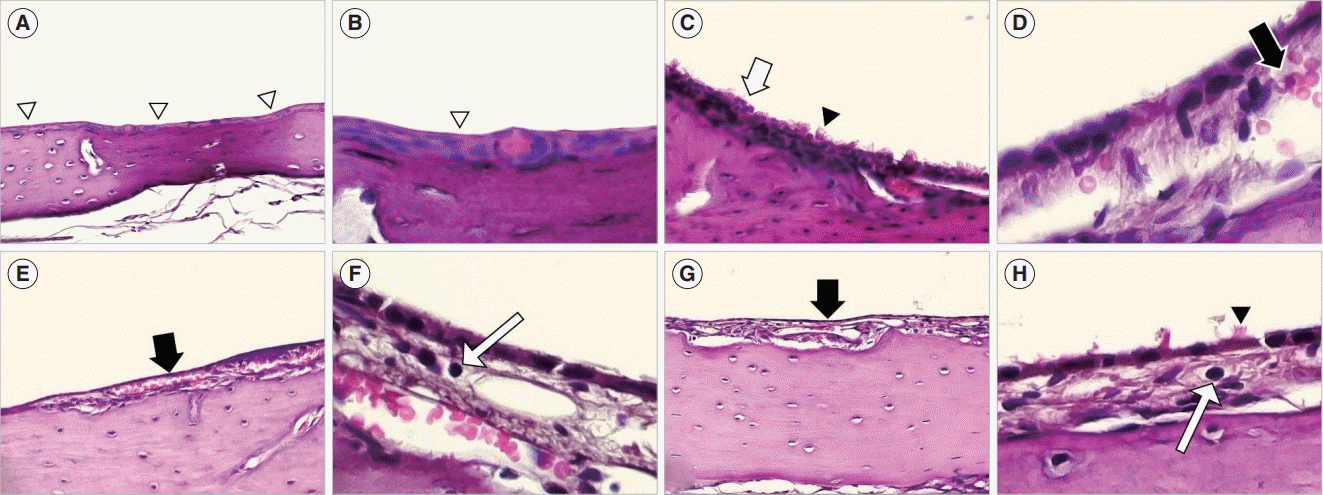 | Fig. 5.Histopathologic findings (H&E) of the middle ear mucosa. In the control group: (A, ×100): Non-keratinized stratified squamous epithelium (arrowheads). (B, ×400): The epithelial layer (arrowhead) shows a dense configuration. In the 3-day exposure group: (C, ×100): Hyperplasia of the stratified squamous cells (arrow) and increased cilia (arrowhead). (D, ×400): Hyperplasia of epithelial cells, thickening of the subepithelial space, and enlargement of angio-capillary tissue (arrow). In the 7-day exposure group: (E, ×100): Increased angio-capillary tissue within the thickened subepithelial tissue (arrow). (F, ×400): Inflammatory cell (lymphocyte) infiltration (arrow). In the 14-day exposure group: (G, ×100): A thickened subepithelial space and increased angio-capillary tissue (arrow). (H, ×400): Increased cilia (arrowhead) and inflammatory cell (lymphocyte) infiltration (arrow). |
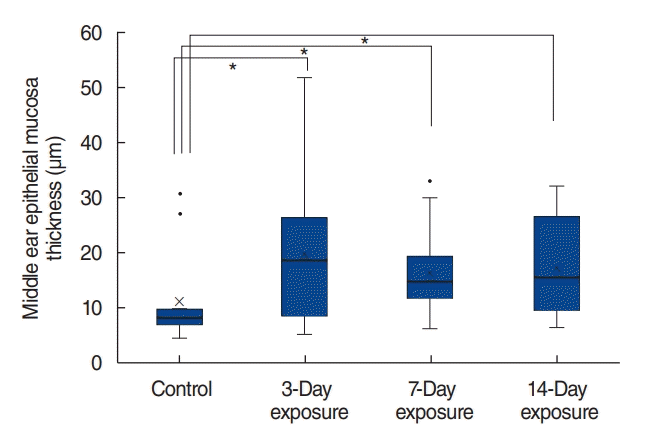 | Fig. 6.Alterations in the thickness of the middle ear mucosa. Compared to the thickness of the middle ear mucosa in the control group, that in the 3-day, 7-day, and 14-day exposure groups significantly increased (P=0.009, P=0.002, and P=0.001, respectively). The duration of particulate matter exposure and the thickness of the mucous membrane did not show a proportional trend. × indicates mean value; horizontal line in the box, median value. *P<0.05. |
Ultrastructural findings of the ET and middle ear mucosa
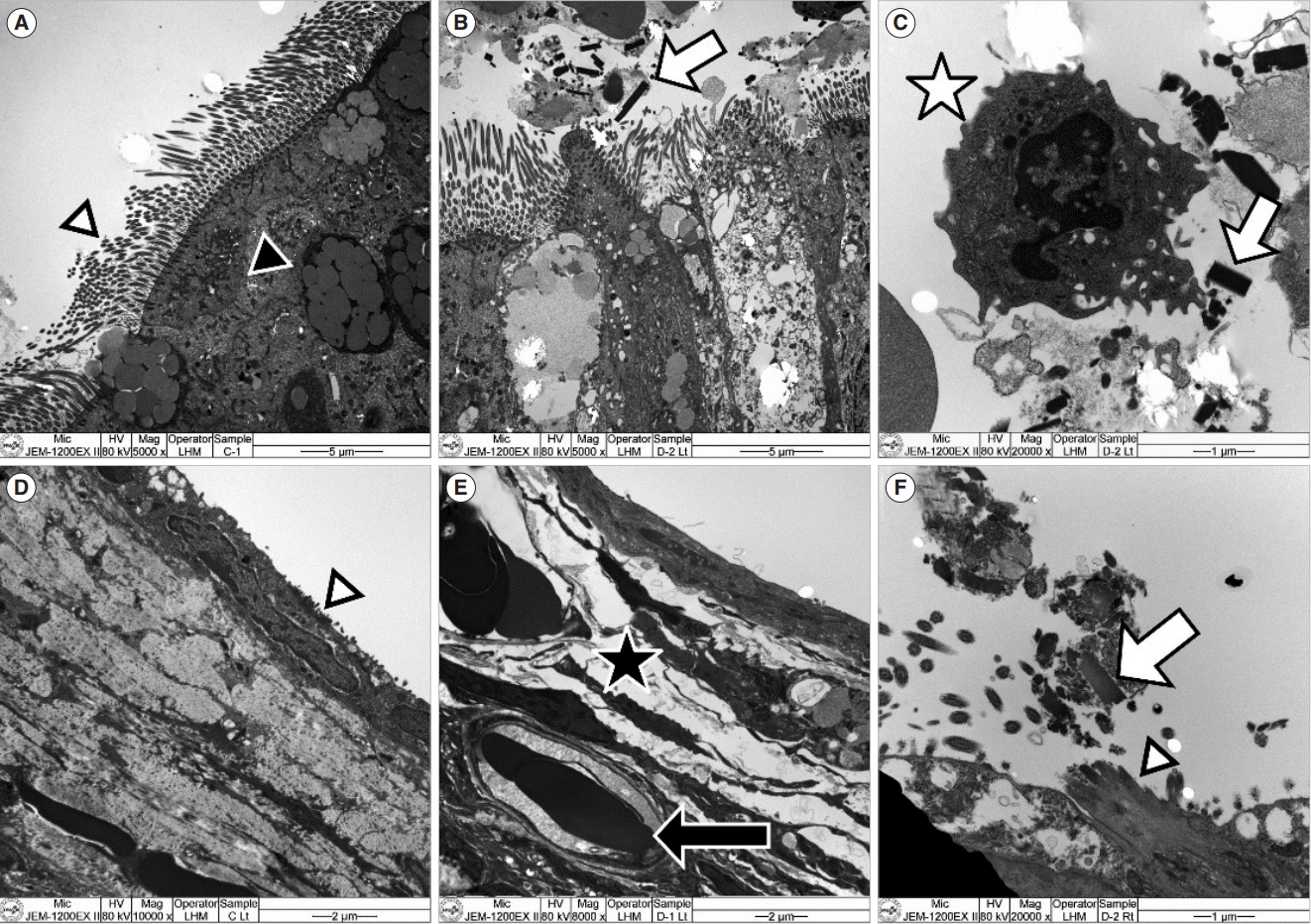 | Fig. 7.Transmission electron microscopy findings of the Eustachian tube (ET) and middle ear mucosa. (A) In the ET mucosa of the control group, cilia (white arrowhead) and goblet cells (black arrowhead) were observed (×5,000). (B) In the ET mucosa of the 3-day exposure group, particulate matter (PM) particles (arrow) were observed over the cilia (×5,000). (C) In the ET mucosa of the 3-day exposure group with higher magnification, a neutrophil (asterisk) and PM particles (arrow) were observed together (×20,000). (D) In the middle ear mucosa of the control group, small ciliary tissue (arrowhead) was observed (×10,000). (E) In the middle ear mucosa of the 3-day exposure group, thickened subepithelial tissue (asterisk) was identified, and erythrocytes (arrow) were observed inside of the angio-capillary tissue (×8,000). (F) In the middle ear mucosa near the ET in the 3-day exposure group, cilia (arrowhead) and PM particles (arrow) over the cilia were observed (×20,000). |
Expression of inflammatory cytokines (IL-1β, IL-6, TNF-α) and VEGF in the middle ear mucosa
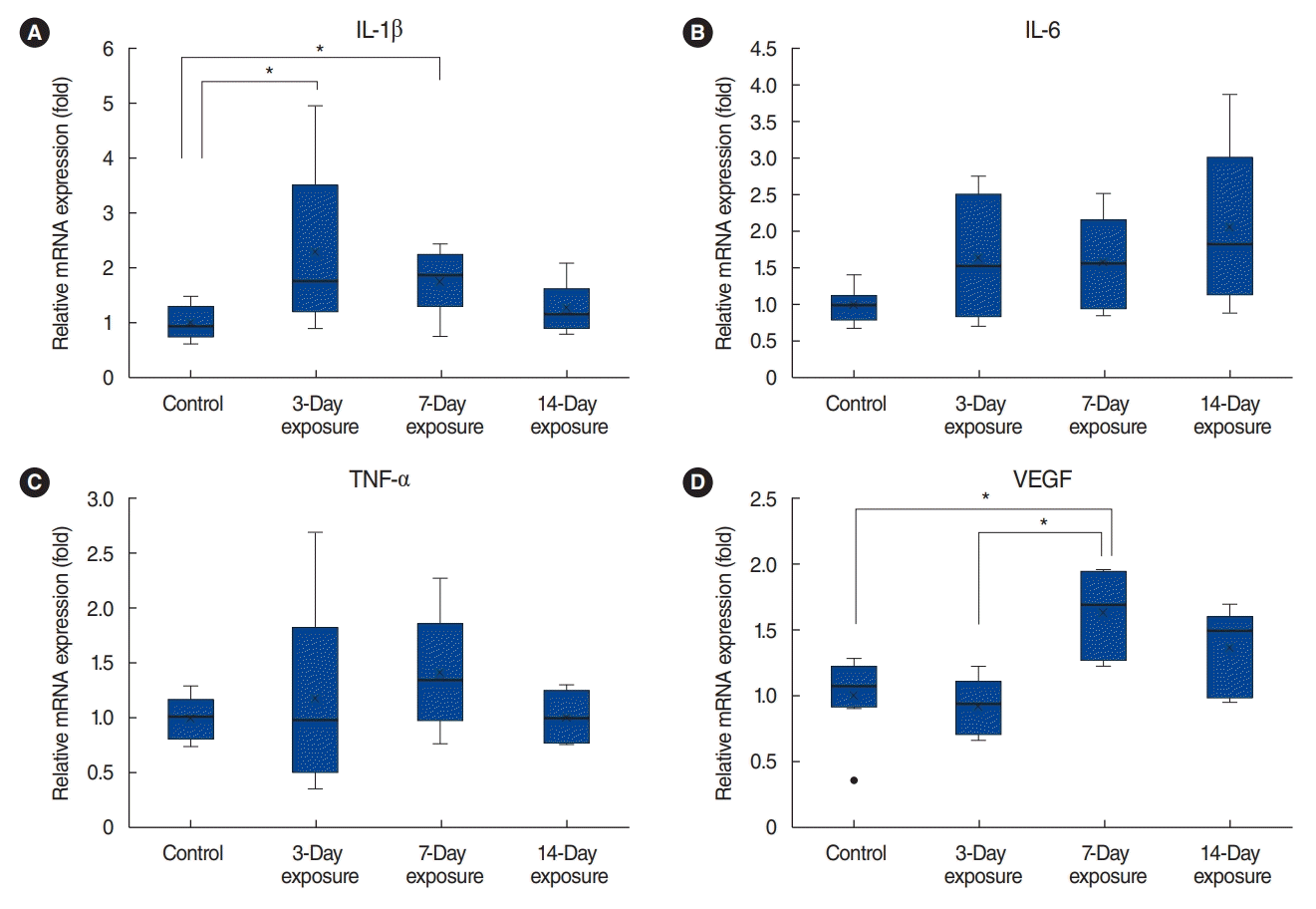 | Fig. 8.Cytokine expression by the control, 3-day, 7-day, and 14-day exposure groups by real-time polymerase chain reaction. The messenger RNA (mRNA) expression of interleukin (IL)-1β (A) significantly increased after particulate matter (PM) exposure in the 3-day and 7-day exposure groups (P=0.035 by the Kruskal-Wallis test). The mRNA expression of IL-6 (B) and tumor necrosis factor (TNF)-α (C) did not significantly increase after PM exposure in the 3-day, 7-day, and 14-day exposure groups (P=0.142 and P=0.472, respectively, by the Kruskal-Wallis test). The mRNA expression of vascular endothelial growth factor (VEGF; D) significantly increased after PM exposure in the 7-day exposure group (P<0.01 by the Kruskal-Wallis test). × indicates mean value; horizontal line in the box, median value. *P<0.05. |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download