Abstract
Objectives.
Ménière disease (MD) is an idiopathic disorder that affects hearing and inner ear balance. Intratympanic gentamicin (ITG) is recognized as an effective treatment for uncontrolled MD characterized by persistent vertigo attacks despite therapy. The video head impulse test (vHIT) and skull vibration-induced nystagmus (SVIN) are validated methods for evaluating vestibular function. A progressive linear relationship has been identified between the slow-phase velocity (SPV) of SVIN determined using a 100-Hz skull vibrator and the gain difference (healthy ear/affected ear) measured by vHIT. The aim of this study was to ascertain whether the SPV of SVIN was associated with the recovery of vestibular function following ITG treatment. Consequently, we sought to determine whether SVIN could predict the onset of new vertigo attacks in patients with MD who were treated with ITG.
Methods.
A prospective longitudinal case-control study was conducted. Several variables were recorded post-ITG and throughout the follow-up period, followed by statistical analyses. Two groups were compared: patients who experienced vertigo attacks 6 months after ITG and those who did not.
Results.
The sample comprised 88 patients diagnosed with MD who underwent ITG treatment. Of the 18 patients who experienced recurring vertigo attacks, 15 demonstrated gain recovery in the affected ear. However, all 18 patients exhibited a decrease in the SPV of SVIN.
Conclusion.
The SPV of SVIN may be more sensitive than vHIT in identifying the recovery of vestibular function following ITG administration. To our knowledge, this is the first study to illustrate the link between a reduction in SPV and the likelihood of vertigo episodes in patients with MD who have been treated with ITG.
Ménière disease (MD) is an idiopathic disorder that impacts hearing and inner ear balance. This disease is marked by episodes of vertigo, a sensation of fullness in the ear, tinnitus, and fluctuating hearing loss. It is thought to be associated with anatomical changes in the inner ear, specifically an increase in the volume of endolymph (the fluid that fills the membranous labyrinth) and a decrease in the volume of perilymph (the fluid that surrounds the membranous labyrinth and fills the bony labyrinth). This shift in the fluid dynamics of the inner ear is referred to as endolymphatic hydrops [1]. The most common treatments for MD involve dietary changes such as a low-salt diet and limited intake of stimulants like caffeine, as well as the administration of systemic or intratympanic corticosteroids. The prevalence of MD ranges from 39 to 190 patients per 100,000 people [2], with 35% and 47% of cases presenting bilaterally over 10 and 20 years, respectively [3].
Intratympanic gentamicin (ITG) is recognized as an effective treatment for uncontrolled MD characterized by persistent vertigo attacks despite ongoing therapy [4,5]. The primary reasons for employing ITG to manage these episodes are its vestibulotoxic effect and its relatively lesser damage to cochlear function compared to other aminoglycosides [6]. The action mechanism of ITG is rooted in its interaction with the peripheral vestibular receptor and its capacity to decrease the gain of the vestibulo-ocular reflex (VOR) for each semicircular canal in the affected ear [7].
The video head impulse test (vHIT) is used to gauge the velocity of the eye during high-frequency and high-velocity cephalic impulses on a specific semicircular canal plane. This allows for the assessment of each canal as an individual entity, enabling the measurement of the angular VOR (aVOR) within its physiological range. Additionally, the vHIT captures refixation saccades that were either unobserved (covert) or observed (overt) when the aVOR was hypofunctional or failed to meet the requirements for head acceleration [8].
vHIT is a validated method for evaluating the function of semicircular canals following ITG application [9]. The relationship between the gain difference in the aVOR of the semicircular canals and the likelihood of a vertigo attack after ITG treatment has been previously established. Therefore, an increase in the gain of the affected ear and a smaller gain difference suggest a higher likelihood of recurrent vertigo attacks and repeated need for ITG [10]. The gain on the affected side is known to deteriorate during the initial 4 weeks of ITG administration. This decrease in gain between 3 and 4 weeks after ITG application may be attributed to the time required for gentamicin to move from the middle to the inner ear, delayed drug clearance, and the gradual progression of cellular damage [11]. In vHIT, a quantity termed the PR score can also be analyzed. Two types of saccades are possible: covert (refixation movements occurring during the head impulse) and overt (those occurring after the head movement has concluded). The saccades signify vestibular recovery, with the level of organization of the response indicating the degree of recovery. In other words, the more overt saccades take place, the less vestibular compensation is present. The PR score is the outcome of an algorithm that assigns a numerical value to the grouping of the saccades. It is based on the coefficient of variation measured at the peak velocity of the eyes. All ocular responses to all impulses recorded in the same trial are calculated. This provides a value between 0 and 100, with values closer to 0 indicating less saccade dispersion [12].
Assessment of skull vibration-induced nystagmus (SVIN) is another method for evaluating vestibular function. This reliable, noninvasive test is simple to administer and can be used to identify the affected side in cases of vestibular deficits, even in chronic and compensated pathologies. It can expose vestibular asymmetry and serves as a vestibular Weber test. The optimal stimulation frequency for this test is 100 Hz [13]. SVIN primarily induces a horizontal nystagmus that beats towards the healthy side in patients with a unilateral vestibular deficit [14]. A direct linear relationship has been observed between the slow-phase velocity (SPV) of SVIN using a 100-Hz skull vibrator and the gain difference (healthy ear/affected ear) as measured on vHIT [15].
The objective of this study was to identify any potential correlation between the SPV of SVIN and the recovery of vestibular function following ITG. Furthermore, we sought to determine whether the SVIN could potentially predict the onset of new vertigo episodes in patients with MD who have undergone ITG treatment.
This prospective longitudinal case-control study was conducted on patients with definite MD who were treated with ITG. The following criteria were used to define definite and probable MD, in accordance with the consensus of the Bárány Society [16]. Definite MD are (1) Two or more spontaneous vertigo attacks, each lasting 20 minutes to 12 hours. (2) Audiometrically documented low- to medium-frequency sensorineural hearing loss in one ear, defined as the affected ear, on at least one occasion before, during, or after one of the episodes of vertigo. (3) Fluctuating aural symptoms (hearing, tinnitus, or fullness) in the affected ear. (4) No other suitable vestibular diagnosis. Probable MD are (1) Two or more episodes of vertigo or dizziness lasting 20 minutes to 24 hours. (2) Fluctuating aural symptoms (hearing, tinnitus, or fullness) in the affected ear. (3) No other suitable vestibular diagnosis.
Our sample included patients with definite MD who were treated with ITG at the Department of ENT and Cervicofacial Pathology of University Hospital of Salamanca, Spain. All patients were assessed by the same otoneurology team. Ethical approval for this study was obtained from the Institutional Review Board of University Hospital of Salamanca (No. PI9610/2017A), and informed consent was waived. We included patients who were diagnosed with definite MD, treated with ITG, and did not present with attacks over the first 6 months after ITG.
The exclusion criteria were as follows: presentation of vertigo attacks within the first 6 months after administration of ITG and spontaneous nystagmus with SPV >2°/sec (to improve the sensitivity of the test, due to the fact that presenting and spontaneous nystagmus may overly artifact the test measurement if it is >2°/sec. We might not identify the component added to the SPV generated by SVIN if it exists).
Follow-up assessment was performed every month up to 6 months after injection with ITG, then every 3 months up to 1 year and then every 6 months up to 1 year more. After that they are seen once a year indefinitely. At each consultation, vHIT and SVIN are performed whether or not they have had crises. Duration of follow-up is undefined. All of our patients treated with ITG will not be discharged.
ITG is considered an effective treatment for uncontrolled MD patients who present persistent vertigo attacks despite treatment. ITG is always injected consistent with the symptoms of the patient, and waiting at least 1 month between injections [5]. The main goal of using ITG is to control the attacks, due to the vestibulotoxic effect of the drug. ITG compared to other aminoglycosides, induces more damage of cochlear function [6]. We consider the patient controlled vertigo attacks in terms of intensity and duration of the attack, when signs of ototoxicity are noticed or when after five injections no improvement is achieved [17]. The Cochrane Collaboration concludes that ITG is an effective treatment to control vertigo in MD, recommending its administration at low doses, at distant intervals of time, according to patient’s symptoms [18]. It is spready administered until attacks are controlled, which is why some patients required more than one injection.
Control of vertigo attacks is due to partial ablation of the vestibular organ. The toxic effect is produced by destruction of hair cells, producing an alteration of the integrity of the plasmatic membrane and preventing the formation of inositol triphosphate IP3, acting intrinsically in the cell releasing free radicals [19]. As we said, we included patients who were diagnosed with definite MD, treated with ITG, and did not present with attacks over the first 6 months after ITG. This 6-month interval was chosen because the response to ITG is considered to stabilize after this period [12]. The dose of ITG used per injection was 0.4–0.5 mL (drug concentration 27 mg/mL).
To perform SVIN, the patient was placed in a seated position with videonystagmoscopy and without makeup. Patients were required to avoid blinking. A skull vibrator with a cylindrical contact at 100 Hz was placed on the mastoid apophysis on either side or perpendicular to the cranial vertex, with a pressure of 10 N for approximately 10 seconds. The tester held the patient’s head with the other hand and measured the SPV of the induced nystagmus [20] using a handheld vibrator Vestibular Vibrator 100 (Synapsis). The SPV was recorded over 10 seconds using VNG software (Ulmer). The SPV of the SVIN for both mastoids was obtained for each patient.
The vHIT was performed using Otometrics ICS Impulse. The patient was seated with goggles secured to the head and fitted with a video camera, sensor, and mirror that reflected the pupil. The device was calibrated for each patient before testing. The patient was asked to stare at a point 1.5 m away. The doctor held the patient’s head and horizontally rotated it in different directions randomly. The pupil was calibrated to cephalic impulses of 10°–20° and 100°–200°/sec. The data recorded met all the necessary quality criteria and included evaluations of speed, consistency, time, groupings, and saccade direction. Gain was also measured (normal: 0.8–1.2; VOR gain=eye velocity/head velocity) [21]. At least 20 impulses were applied to each side, which was sufficient to perform the test according to the literature, and the records were reviewed and maintained manually to prevent artifacts. The most common artifacts in this test include the head overshoot, which is associated with a significantly higher velocity, longer duration, and lower impulse amplitude and consequently leads to a higher saccade latency and lower saccade amplitude; eye blinking, which is associated with a higher number of saccades; and overshoot, which increases the probability of an impulse being located in the atypical gain and velocity ranges [22]. Other common artifacts include those caused by poor calibration of the device, lack of differentiation between refixation saccades and spontaneous nystagmus, manual movements of the goggles or loose goggle straps, head movements when the jaw is being held, head bouncing after the impulse ends, impulses that are not bell-shaped, alterations in pupil tracking that may be affected by light or the corneal reflex, malformations, and ptosis of the eyelid [23].
Because the calculation method for each vHIT device is different, instead of using the vHIT asymmetry criterion to compare the records, we measured the gain difference, since this approach yielded test results that were comparable across all vHIT devices.
The following variables were recorded: sex, age, and affected side. The duration of the disease prior to ITG administration, the number of previous intratympanic corticosteroid injections, and the number of Tumarkin crises and number of ITG injections were also registered. We measured the following variables 6 months after administration of gentamicin and during the follow-up period and compared them: average gain measured with vHIT on the affected and healthy sides, gain difference between the two sides, presence of spontaneous nystagmus, the SPV of the SVIN on the affected and healthy sides, and the modification of the SPV (that is the difference measured in % between the SPV 6 months after ITG and during follow-up), and PR score of the affected side (horizontal canals) [24]. We also recorded new vertigo attacks appearing 6 months after ITG and the interval between administration of gentamicin and the attack. The followup protocol included visits every 6 monthss, while cases showing a new crisis were followed-up in the unit within a week. The follow-up period varied between patients because at the time of data collection, some patients had been monitored for longer periods than others.
Statistical analyses were performed using IBM SPSS 21.0 (IBM Corp.). Two groups were compared, those who presented vertigo attacks after the 6-month interval from ITG, and those who did not. A descriptive study of the sample was conducted and was followed by an analytical study. The chi-square test was used for assessment of qualitative variables; Student t-test was used for assessment of qualitative and quantitative variables; and linear regression, Pearson coefficient, and correlation analyses were used for evaluation of quantitative variables. Logistic regression analysis was used to establish the relationship between the qualitative and quantitative variables. Statistical significance was defined as P<0.05.
This descriptive frequency study included a study population of 88 patients with definite MD who were treated with ITG. The average age of the patients experiencing vertigo attacks (group A) was 55 years, while that of patients without such episodes (group B) was 59 years (Table 1). The statistical analyses revealed no significant associations between groups regarding sex, age, affected side, prior use of intratympanic corticoids, number of ITG injections, or Tumarkin crises.
Statistically significant correlations (P<0.05) were observed between the gain on the affected side and the gain difference between the healthy and affected sides, and the PR score after 6 months and throughout the follow-up period (Table 1). The differences in gain between the healthy and affected ears at 6 months, presented as mean±standard deviation, were 0.59±0.05 in group A and 0.52±0.08 in group B. Subsequent follow-up assessments revealed gain differences of 0.16±0.06 in group A and 0.34±0.06 in group B. Of the 18 patients in group A, 15 exhibited gain recovery in the affected ear. However, none of the 70 patients in group B demonstrated recovery in the gain of the affected ear (Fig. 1).
Statistically significant associations (P<0.05) were observed between the SPV of the affected and healthy sides, as well as the alterations in SPV observed after 6 months and throughout the follow-up period (Table 1). In group A, the SPV on the affected side showed a change of 37%±12%, while in group B, it changed by 0.8%±5.0%. After 6 months, the SPV on the affected side was measured at 9.0°/sec±2.8°/sec in group A and 9.6°/sec±2.4°/sec in group B. In group A, a reduction in the SPV of the SVIN was observed in all 18 patients (Fig. 1).
A positive correlation was observed between the changes in SPV on the affected side during follow-up and the gain on the same side (Pearson correlation coefficient, 0.6; P=0.000). A statistically significant relationship was found between the gain difference during follow-up and the change in SPV (P=0.000; Pearson correlation coefficient, 0.7). A linear regression model using these variables demonstrated a positive linear relationship. We observed a progressive linear relationship, with a Pearson correlation coefficient of 0.6 (P=0.000), of SPV and its changes with PR. The linear regression model that compared the changes in SPV and the difference in gain during follow-up revealed a linear relationship (Fig. 2).
We utilized a logistic regression model to examine the correlation between the occurrence of vertigo crises following ITG administration and several variables: SPV on the affected side, alterations in SPV, gain on the affected side, and discrepancies between gains. A statistically significant relationship (P<0.01) was identified between these variables and the incidence of crises post-ITG administration.
SVIN assessment is a straightforward clinical test that involves applying a vibrator with a frequency of 100 Hz and moderate intensity, roughly equivalent to a body massager, to either mastoid of a patient suffering from total unilateral vestibular loss. This induces a primarily horizontal nystagmus, with clinically evident rapid phases moving away from the affected side [13]. The nystagmus stops immediately once the vibration is removed, with no subsequent nystagmus observed. Video recordings reveal that the nystagmus consists of SPV deviations away from the healthy ear, interspersed with quick return phases directed away from the affected ear [25]. These rapid phases can be easily detected by a clinician at the bedside using Frenzel glasses, although quantifying SPV necessitates three-dimensional recordings. Interestingly, with SVIN, the direction of the nystagmus remains the same regardless of which mastoid is stimulated. However, when the same procedure is performed on healthy individuals, it does not consistently induce nystagmus with an SPV greater than 2.5°/sec [13].
The clinical interpretation of these findings can be summarized as follows: an SVIN at 100 Hz, with the same direction when both mastoids are stimulated, suggests an asymmetry in the function of the semicircular canals between the two labyrinths. The fast phase points to the labyrinth with diminished function [26]. Three-dimensional recordings [13] have demonstrated that when mastoid vibration is applied to patients with a unilateral vestibular deficit, the SVIN exhibits a horizontal and torsional component directed towards the side of the healthy ear. Furthermore, evidence suggests that mastoid vibration simultaneously activates all semicircular canals and otoliths [27].
Recordings of vestibular afferents in guinea pigs have demonstrated that a 100-Hz vibration effectively stimulates both the semicircular canals and otolithic organs, resulting in irregular resting discharges. These discharges are termed “irregular” due to the variability in the interval between action potentials in these neurons when no stimulation is present (that is, during the resting discharge). These irregular afferents innervate the amphora-shaped type I receptors located in the crista crest or striola of the otolithic maculae [28]. Notably, however, gentamicin exhibits a predilection for ototoxicity in type I hair cells (HC-I) [29]. The relationship between the SPV measurement of the SVIN and the disparity in gain (healthy side versus affected side) as determined by the vHIT has been previously documented [15]. In patients with a unilateral vestibular deficit, the test has a sensitivity of 98%. However, it yields negative results in patients exhibiting hypoexcitability on the healthy side, as measured by the caloric test [30].
The findings from this study support the observed decrease in gain measured by the vHIT following gentamicin injection. They also highlight the correlation between gain recovery and the increase in the PR score on the affected side, as well as the recurrence of vertigo attacks. When patients exhibit ungrouped saccades, the PR score increases, indicating vestibular decompensation in those who experience recurring vertigo attacks. In contrast, in the group without recurrent episodes, the PR score decreased, suggesting vestibular compensation and a lower likelihood of vertigo recurrence [12]. Previous research has shown that the frequency of recurring episodes is significantly lower if the VOR gain has been reduced by less than 33% from the baseline after the first application of ITG [31]. This underscores the importance of monitoring VOR changes in patients undergoing ITG treatment. vHIT serves as a practical and straightforward tool for this purpose. In contrast, several studies have confirmed the spontaneous regeneration of the vestibular system following ototoxic damage in mammals [32]. Additionally, histological studies have shown the spontaneous regeneration of hair cells after ITG [33].
The findings of our study are consistent with previous research. However, we also discovered that monitoring the SPV in SVIN can be as beneficial as measuring VOR gains with vHIT. Specifically, in patients with MD treated with ITG who experienced new vertigo episodes, the gain in the affected ear was restored in 15 of 18 cases. Moreover, the SPV of the SVIN had decreased before the crisis in all 18 cases. This could suggest that SVIN is particularly sensitive and highly valuable for predicting the recurrence of vertigo attacks in MD after treatment with ITG.
The increased sensitivity of the SPV of SVIN compared to vHIT in patients experiencing vertigo crises could be attributed to the fact that gentamicin preferentially affects HC-I cells, which are specifically stimulated by SVIN [23]. Research has shown that if the VOR gain difference in the horizontal canal is relatively low following initial ITG treatment, the patient may experience poor control over vertigo [10].
Qian et al. [29] conducted an in vivo study combining two antibody markers, demonstrating that HC-I cells in all subdivisions of the utricular macule showed a greater capacity for gentamicin uptake than type II hair cells (HC-II). This observation aligns with previous findings indicating that HC-I cells are the primary targets of gentamicin and are more susceptible to its effects than HC-II cells [7]. Several reasons contribute to the higher accumulation of gentamicin in vivo in HC-I compared to HCII cells. First, the development of HC-I precedes that of HC-II cells [34]. This suggests that the mechanisms for gentamicin uptake are likely established in HC-I before HC-II. Second, gentamicin can penetrate hair cells either through the mechanoelectrical transduction channel on the stereocilia [35] or via apical endocytosis [36]. Given that HC-I hair bundles are more voluminous, they offer a greater number of mechanoelectrical transduction channels, resulting in a larger transduction current [37]. Third, HC-I cells in the cristae of the semicircular canals have higher negative resting potentials than HC-II cells [38]. This leads to a larger electrochemical gradient across the apical membrane, which is thought to provide an electromotive force for gentamicin entry into the HC [35]. In contrast, the utricular maculae are anatomically divided into striolar and extrastriolar regions based on the HC density and morphology. In the striola, HCs are less densely packed, and proportionally more HC-I cells are present; in contrast the number of both types of HC are approximately equivalent in the extrastriola [39].
Other analyses [26] have demonstrated a near-total loss of HC-I cells, with a 94% loss in the crista ampullaris of the lateral semicircular canal and an 86% loss in the utricular macula. In contrast, the loss of HC-II cells has been minimal, with a 4% loss in the crista ampullaris of the lateral semicircular canal and a 6% loss in the utricular macula. In a separate study, Hirvonen et al. [40] used a chinchilla model and filled the tympanic cavity with ITG. This resulted in a 57% reduction in the overall density of hair cells, including a 99% loss of HC-I and a 36% loss of HC-II cells.
In contrast, recordings from individual vestibular afferents in guinea pigs have demonstrated that low-frequency skull vibration (100 Hz) effectively stimulates the semicircular canal and otolith neurons that exhibit irregular resting discharge. These afferents are termed “irregular” due to the variability in the interval between action potentials when no stimulation is exerted, also known as the resting discharge. These irregular afferents innervate the amphora-shaped type I receptors located at the crest of the crista or at the striola of the otolithic maculae [9,10]. They respond with high sensitivity to 100-Hz vibration. Conversely, other afferent neurons, which primarily synapse on type II receptors on the crista slopes or in the extrastriolar area of the otolithic maculae, exhibit a regular resting discharge. However, they have a very poor or even non-existent response to vibration at clinically safe intensities [4].
To our knowledge, this is the first study to describe the association between decreased SPV and the likelihood of vertigo episodes in patients with MD who have been treated with ITG. Recovery in gain and a reduction in the SPV of SVIN are good predictors of new vertigo attacks after ITG administration. The observed preference of gentamicin for HC-I cells, coupled with the primary stimulation of these cells by SVIN, likely explains why the SPV of SVIN may be more effective than vHIT in detecting vestibular function recovery following ITG administration. However, additional studies are required to confirm this hypothesis.
▪ Among 18 patients who experienced recurrent vertigo attacks, 15 demonstrated gain recovery, as measured by a video head impulse test (vHIT) of the affected ear.
▪ All 18 patients exhibited a decrease in the slow-phase velocity (SPV) of skull vibration-induced nystagmus (SVIN).
▪ The SPV of SVIN may be superior to vHIT for identifying the recovery of vestibular function after the administration of intratympanic gentamicin (ITG).
▪ To our knowledge, this is the first study to describe the association between decreased SPV and the likelihood of vertigo attacks in patients with Ménière disease treated with ITG.
REFERENCES
1. Wright T. Meniere’s disease. BMJ Clin Evid. 2015; Nov. 2015:0505.
2. Tyrrell JS, Whinney DJ, Ukoumunne OC, Fleming LE, Osborne NJ. Prevalence, associated factors, and comorbid conditions for Meniere’s disease. Ear Hear. 2014; Jul-Aug. 35(4):e162–9.
3. Huang CH, Young YH. Bilateral Meniere’s disease assessed by an inner ear test battery. Acta Otolaryngol. 2015; Mar. 135(3):233–8.
4. Pullens B, van Benthem PP. Intratympanic gentamicin for Meniere’s disease or syndrome. Cochrane Database Syst Rev. 2011; Mar. (3):CD008234.
5. Junet P, Karkas A, Dumas G, Quesada JL, Schmerber S. Vestibular results after intratympanic gentamicin therapy in disabling Meniere’s disease. Eur Arch Otorhinolaryngol. 2016; Oct. 273(10):3011–8.
6. Anagnostou E, Koutsoudaki P, Stavropoulos A, Evdokimidis I. Effect of common antivertiginous agents on the high velocity vestibulo-ocular reflex. Clin Neurophysiol. 2017; Nov. 128(11):2211–6.
7. Lyford-Pike S, Vogelheim C, Chu E, Della Santina CC, Carey JP. Gentamicin is primarily localized in vestibular type I hair cells after intratympanic administration. J Assoc Res Otolaryngol. 2007; Dec. 8(4):497–508.
8. Cordero-Yanza JA, Arrieta Vazquez EV, Hernaiz Leonardo JC, Mancera Sanchez J, Hernandez Palestina MS, Perez-Fernandez N. Comparative study between the caloric vestibular and the video-head impulse tests in unilateral Meniere’s disease. Acta Otolaryngol. 2017; Nov. 137(11):1178–82.
9. Marques PS, Dias CC, Perez-Fernandez N, Spratley J. Instrumental head impulse test changes after intratympanic gentamicin for unilateral definite Meniere’s disease: a systematic review and meta-analysis. Auris Nasus Larynx. 2018; Oct. 45(5):943–51.
10. Lee JY, Kim MB. Change of VOR gain and pure-tone threshold after single low-dose intratympanic gentamicin injection in Meniere’s disease. Acta Otolaryngol. 2020; Apr. 140(4):314–8.
11. Martin-Sanz E, Diaz JY, Esteban-Sanchez J, Sanz-Fernandez R, Perez-Fernandez N. Delayed effect and gain restoration after intratympanic gentamicin for Meniere’s disease. Otol Neurotol. 2019; Jan. 40(1):79–87.
12. Rey-Martinez J, Batuecas-Caletrio A, Matino E, Perez Fernandez N. HITCal: a software tool for analysis of video head impulse test responses. Acta Otolaryngol. 2015; Sep. 135(9):886–94.
13. Dumas G, Curthoys IS, Lion A, Perrin P, Schmerber S. The skull vibration-induced nystagmus test of vestibular function: a review. Front Neurol. 2017; Mar. 8:41.
14. Sanchez-Blanco C, Yanez-Gonzalez R, Batuecas-Caletrio A. Nistagmo inducido por vibración en Otorrinolaringología. Rev ORL. 2018; 9(3):221–6.
15. Batuecas-Caletrio A, Martinez-Carranza R, Garcia Nunez GM, Fernandez Nava MJ, Sanchez Gomez H, Santacruz Ruiz S, et al. Skull vibration-induced nystagmus in vestibular neuritis. Acta Otolaryngol. 2020; Dec. 140(12):995–1000.
16. Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandala M, et al. Diagnostic criteria for Meniere’s disease according to the Classification Committee of the Barany Society. HNO. 2017; Nov. 65(11):887–93.
17. Villaoslada-Fuentes C, Fernandez-Nava MJ, Ferreira-Cendon S, Villaoslada-Fuentes R, Sanchez-Gomez H, Batuecas-Caletrio A. Pérdida de audición tras gentamicina intratimpánica en la Enfermedad de Ménière. Estudio retrospectivo. Rev ORL. 2021; Jul/Sep. 12(3):243–52.
18. Diamond C, O’Connell DA, Hornig JD, Liu R. Systematic review of intratympanic gentamicin in Meniere’s disease. J Otolaryngol. 2003; Dec. 32(6):351–61.
19. Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000; Jan-Feb. 5(1):3–22.
20. Dumas G, Perrin P, Ouedraogo E, Schmerber S. How to perform the skull vibration-induced nystagmus test (SVINT). Eur Ann Otorhinolaryngol Head Neck Dis. 2016; Nov. 133(5):343–8.
21. Halmagyi GM, Chen L, MacDougall HG, Weber KP, McGarvie LA, Curthoys IS. The video head impulse test. Front Neurol. 2017; Jun. 8:258.
22. Trinidad-Ruiz G, Rey-Martinez J, Matino-Soler E, Batuecas-Caletrio A, Martin-Sanz E, Perez-Fernandez N. Relevance of artifact removal and number of stimuli for video head impulse test examination. Ear Hear. 2020; Sep/Oct. 41(5):1397–406.
23. Mantokoudis G, Saber Tehrani AS, Kattah JC, Eibenberger K, Guede CI, Zee DS, et al. Quantifying the vestibulo-ocular reflex with videooculography: nature and frequency of artifacts. Audiol Neurootol. 2015; 20(1):39–50.
24. Batuecas-Caletrio A, Rey-Martinez J, Trinidad-Ruiz G, Matino-Soler E, Cruz-Ruiz SS, Munoz-Herrera A, et al. Vestibulo-ocular reflex stabilization after vestibular Schwannoma surgery: a story told by Saccades. Front Neurol. 2017; Jan. 8:15.
25. Curthoys IS. Generation of the quick phase of horizontal vestibular nystagmus. Exp Brain Res. 2002; Apr. 143(4):397–405.
26. Curthoys IS. The neural basis of skull vibration induced nystagmus (SVIN). Audiol Res. 2021; Oct. 11(4):557–66.
27. Cohen B, Suzuki JI, Bender MB. Eye movements from semicircular canal nerve stimulation in the cat. Ann Otol Rhinol Laryngol. 1964; Mar. 73:153–69.
28. Fernandez C, Goldberg JM, Baird RA. The vestibular nerve of the chinchilla. III. Peripheral innervation patterns in the utricular macula. J Neurophysiol. 1990; Apr. 63(4):767–80.
29. Qian X, He Z, Wang Y, Chen B, Hetrick A, Dai C, et al. Hair cell uptake of gentamicin in the developing mouse utricle. J Cell Physiol. 2021; Jul. 236(7):5235–52.
30. Dumas G, Perrin P, Schmerber S. Nystagmus induced by high frequency vibrations of the skull in total unilateral peripheral vestibular lesions. Acta Otolaryngol. 2008; Mar. 128(3):255–62.
31. Miller MW, Agrawal Y. Intratympanic Therapies for Meniere’s disease. Curr Otorhinolaryngol Rep. 2014; Sep. 2(3):137–43.
32. Cotanche DA, Lee KH. Regeneration of hair cells in the vestibulocochlear system of birds and mammals. Curr Opin Neurobiol. 1994; Aug. 4(4):509–14.
33. Bremer HG, Versnel H, Hendriksen FG, Topsakal V, Grolman W, Klis SF. Does vestibular end-organ function recover after gentamicin-induced trauma in Guinea pigs. Audiol Neurootol. 2014; 19(2):135–50.
34. McInturff S, Burns JC, Kelley MW. Characterization of spatial and temporal development of Type I and Type II hair cells in the mouse utricle using new cell-type-specific markers. Biol Open. 2018; Nov. 7(11):bio038083.
35. Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol. 2005; Sep. 567(Pt 2):505–21.
36. Hashino E, Shero M, Salvi RJ. Lysosomal augmentation during aminoglycoside uptake in cochlear hair cells. Brain Res. 2000; Dec. 887(1):90–7.
37. Denk W, Holt JR, Shepherd GM, Corey DP. Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron. 1995; Dec. 15(6):1311–21.
38. Chen JW, Eatock RA. Major potassium conductance in type I hair cells from rat semicircular canals: characterization and modulation by nitric oxide. J Neurophysiol. 2000; Jul. 84(1):139–51.
39. Desai SS, Zeh C, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol. 2005; Jan. 93(1):251–66.
40. Hirvonen TP, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol. 2005; Feb. 93(2):643–55.
Fig. 1.
Box and whisker plots. In the group that did not experience vertigo attacks after intratympanic gentamicin (“no”, group B), no change was noted in the gain on the affected side over time (A), nor was any change observed in the slow-phase velocity (SPV) (B). The SPV modification percentage remained close to 0% (C). Conversely, among those who did experience subsequent attacks (“yes”, group A, blue), the SPV decreased by 37% (C), the gain on the affected side recovered (A), and the SPV also decreased (B). For all patients in group A, the gain difference decreased because the gain on the affected side recovered in every case. The SPV also decreased in all patients in group A. VIN, vibration-induced nystagmus.
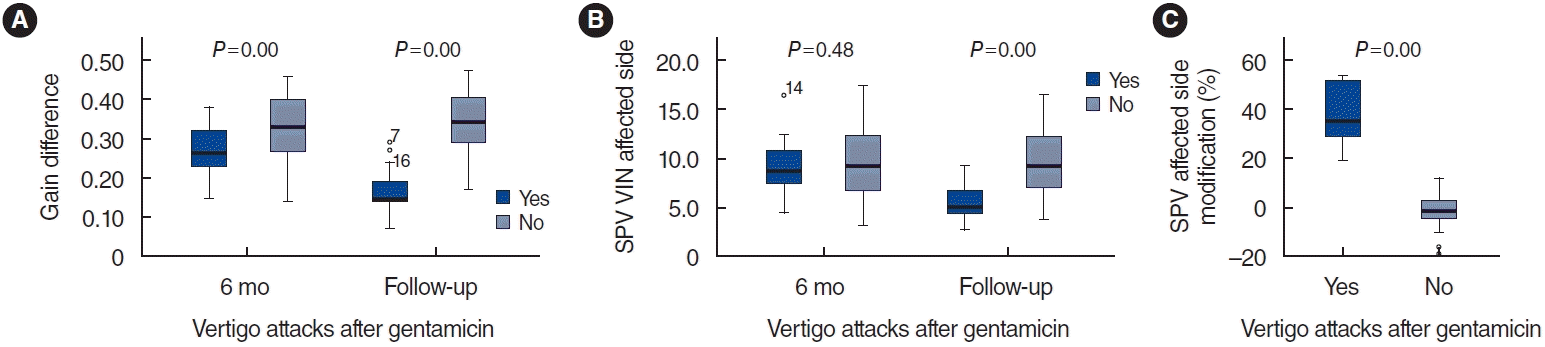
Fig. 2.
Linear regression model. The blue dots denote patients who experienced vertigo attacks following intratympanic gentamicin (ITG) (“yes”, group A), while the green dots represent those who did not (“no”, group B). (A) As the gain in the affected ear increases, the slow-phase velocity (SPV) decreases (as evidenced by the predominance of blue dots in the lower right quadrant). This demonstrates a correlation between the SPV of the diseased side and the gain on that same side during follow-up (post-ITG). The Pearson correlation coefficient was 0.6, with a P-value of 0.000. (B) In the blue group, as the gain in the diseased ear recovered and the disparity in gains between both ears diminished, the SPV exhibited a greater percentage of change. In other words, it slowed down compared to the pre-crisis SPV. However, in the green group, the SPV remained unchanged. The Pearson correlation coefficient was 0.7, with a P-value of 0.000. VIN, vibration-induced nystagmus.
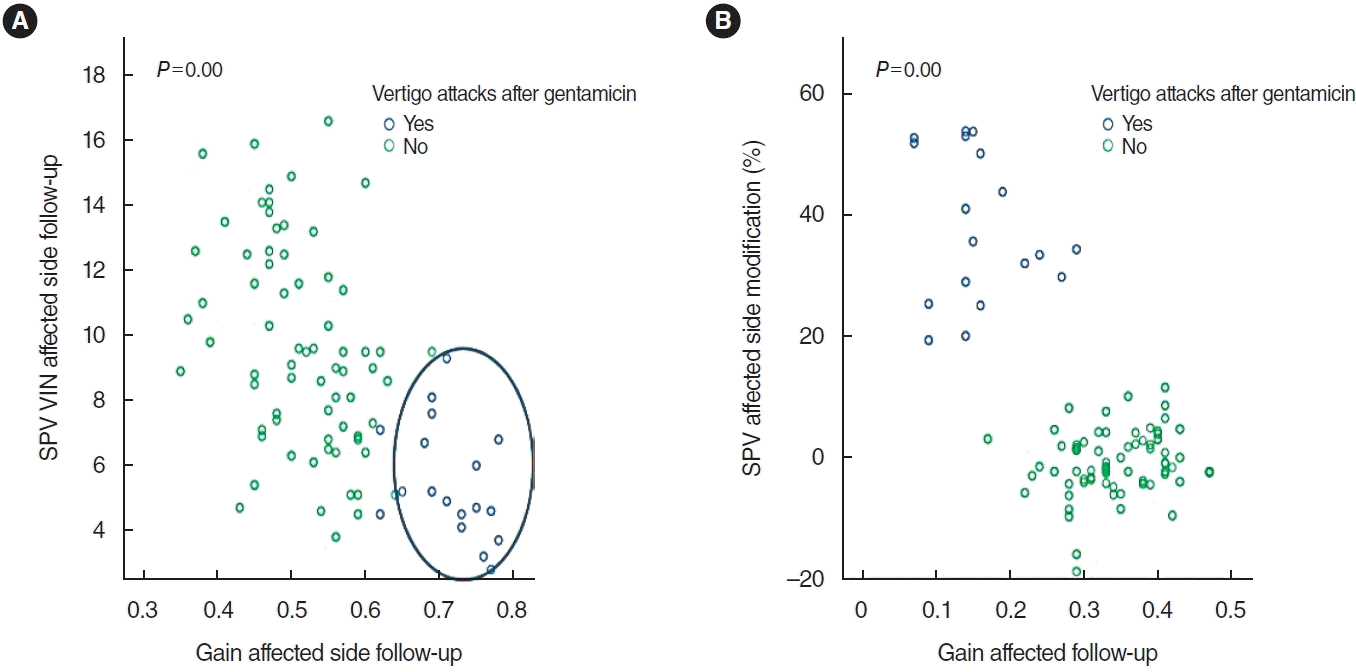
Table 1.
Sample analysis




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download