Abstract
Obstructive sleep apnea (OSA) is a common disorder characterized by upper airway obstruction during sleep. To reduce the morbidity of OSA, sleep specialists have explored various methods of managing the condition, including manifold positive airway pressure (PAP) techniques and surgical procedures. Nasal obstruction can cause significant discomfort during sleep, and it is likely that improving nasal obstruction would enhance the quality of life and PAP compliance of OSA patients. Many reliable studies have offered evidence to support this assumption. However, few comprehensive guidelines for managing OSA through nasal surgery encompass all this evidence. In order to address this gap, the Korean Society of Otorhinolaryngology-Head and Neck Surgery (KORL-HNS) and the Korean Society of Sleep and Breathing designated a guideline development group (GDG) to develop recommendations for nasal surgery in OSA patients. Several databases, including OVID Medline, Embase, the Cochrane Library, and KoreaMed, were searched to identify all relevant papers using a predefined search strategy. The types of nasal surgery included septoplasty, turbinate surgery, nasal valve surgery, septorhinoplasty, and endoscopic sinus surgery. When insufficient evidence was found, the GDG sought expert opinions and attempted to fill the evidence gap. Evidence-based recommendations for practice were ranked according to the American College of Physicians’ grading system. The GDG developed 10 key action statements with supporting text to support them. Three statements are ranked as strong recommendations, three are only recommendations, and four can be considered options. The GDG hopes that this clinical practice guideline will help physicians make optimal decisions when caring for OSA patients. Conversely, the statements in this guideline are not intended to limit or restrict physicians’ care based on their experience and assessment of individual patients.
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder characterized by repeated episodes of partial or complete upper airway collapse during sleep. The prevalence of OSA is reported to be 3%–9% in the general population [1,2]. OSA is associated with resistant hypertension, cardiovascular disease, neurological disease, and mortality from various causes [3-7]. The social costs of sleep disorders have increased over the years [8]. Specifically, there has been growing social concern regarding healthy sleep due to traffic accidents caused by daytime sleepiness, a significant sleep apnea symptom, and large-scale disasters caused by a lack of attention [9-11].
Many societies worldwide, including the American Academy of Sleep Medicine (AASM), recommend using positive airway pressure (PAP) devices as a first-line treatment for OSA [12]. However, studies of PAP device adherence rates have presented varying results [13-15]. Most studies reported a high non-adherence rate to PAP device use; according to Weaver and Grunstein [14], the non-adherence rate to continuous PAP (CPAP) was 83%, using the defined value of adherence to the AASM minimum acceptable usage standards. Therefore, various methods to increase PAP adherence are being studied, and sleep surgery as an alternative treatment is also being discussed. Among the surgical options, nasal surgery is logically possible as an alternative surgical modality while increasing adherence to PAP [16,17].
Many patients with OSA report experiencing nasal obstruction, a known risk factor for sleep-disordered breathing [18-21]. In addition to causing sleeping discomfort, nasal obstruction physiologically increases airflow resistance in the upper airway, potentially lowering intraluminal pressure in the pharynx and leading to pharyngeal closure. Therefore, this negative process can increase sleep apnea severity or the applied pressure on the PAP device [22]. However, mixed results have been reported regarding whether nasal surgery to overcome these processes positively affects OSA or PAP adherence [23,24]. The alarmingly high non-adherence rate for nasal PAP devices should be of great interest to clinicians because low adherence to PAP device use can reduce the therapeutic effect and increases the risk of complications of OSA [25]. Steady progress has been made in research on surgical interventions to treat OSA. In particular, there have been several studies on nasal surgery to solve nasal congestion. Although nasal surgery as a sole intervention is generally insufficient as a curative treatment modality for OSA, nasal surgery effectively reduces PAP device pressure settings [26,27].
Despite the importance of nasal surgery to increase PAP compliance or as a therapeutic method to address OSA, guidelines for consistent medical practice and patient education have not been established or published. Therefore, the target population for this guideline is patients ≥18 years of age with a clinical diagnosis of OSA. We defined OSA patients as those with an apnea-hypopnea index (AHI) of ≥5 points according to polysomnography (PSG).
The guideline aims to improve the quality of decision-making about whether to perform nasal surgery in patients with OSA and to provide definite and practicable recommendations for implementing this decision in clinical practice. The guideline aims to contribute to effective disease management by increasing the effectiveness of nasal surgery in treating nasal and sleep-related manifestations in patients with OSA. It also seeks to improve PAP adherence in OSA patients who do not tolerate PAP therapy due to nasal obstruction. This guideline is intended for review by all sleep specialists who may treat OSA and recommend nasal surgery to patients or refer patients to an otorhinolaryngologist. It should be noted that PAP is the standard treatment for moderate to severe OSA, and this guideline does not recommend treating OSA with nasal surgery alone. Instead, this guideline systematically summarizes the benefits of nasal surgery for OSA patients with nasal obstruction who have not experienced symptom improvement with medical therapy. Therefore, this guideline can be applied to provide a rationale for either performing nasal surgery or referring adult OSA patients with nasal obstruction and unresponsive symptoms to an otorhinolaryngologist, regardless of the setting. This includes adult OSA patients on PAP therapy who have nasal obstruction affecting their PAP adherence, if their symptoms do not improve with medical therapy. However, it is not intended for use in pediatric patients or those in whom multi-level surgery is indicated. To the best of our knowledge, there is limited evidence that the efficacy of nasal surgery in managing nasal and sleep-related symptoms in children and adolescents with OSA is similar to that in adults with OSA. Therefore, child and adolescent patients were excluded from the relevant subjects because the literature searched and cited to develop this guideline included only adults. Additionally, since this guideline seeks to aid in decision-making for nasal surgery by considering evidence of the effectiveness of nasal surgery alone in patients with OSA, papers involving other surgical procedures performed for OSA concurrently with nasal surgery were excluded from this work. Other types of surgery for OSA include tonsillectomy, uvulopalatopharyngoplasty, uvulopalatal flap, expansion sphincter pharyngoplasty, palatal muscle resection, palatal implants, genioglossus advancement, and reduction procedures for the tongue (such as radiofrequency ablation, glossectomy, and transoral robotic surgery). Hypoglossal nerve stimulation and maxillomandibular advancement are also options.
OSA is a common sleep disease that can cause serious health problems and huge economic costs. The prevalence rates of OSA (AHI ≥5 points) in the United States (Wisconsin sleep cohort) were 24% and 9% in men and women aged 30–60 years, respectively [26]. When the criteria of an AHI ≥5 points with symptoms such as daytime sleepiness were applied, the prevalence of OSA was 4% in men but 2% in women [26]. In Korea, the prevalence of OSA was reported to be 27% in men or 16% in women based on an AHI ≥5 points and 4.5% in men or 3.2% in women based on the criteria of an AHI ≥5 points with daytime sleepiness, respectively [27]. According to a recent study estimating the worldwide prevalence and burden of OSA, approximately 1 billion (936 million) adults aged 30–69 years globally were calculated to have OSA using the criterion of an AHI ≥5 points, regardless of symptoms [8]. Of these, 425 million (>45%) have moderate to severe OSA (AHI ≥15 points), requiring aggressive management [8]. If left untreated, OSA can lead to several medical complications and increase the incidence of accidents and total mortality. OSA has been associated with an increased risk for hypertension (odds ratio [OR], 2.9), heart failure (relative risk [RR], 2.4), type 2 diabetes (OR, 1.6), stroke (OR, 3.8), motor vehicle accidents (RR, 2.4), occupational accidents (RR, 2.2), and death in severe OSA (hazard ratio, 3.8) [28]. Many studies have indicated that undiagnosed OSA patients spend more on total healthcare usage than patients without OSA [29-33]. Specifically, patients with undiagnosed OSA pay $1,950–$3,899 annually more than those without OSA [28-33]. In addition, OSA patients managed with PAP pay $2,700–$5,200 less annually than those with untreated OSA [28,30,31]. A white paper from the AASM reported that OSA affected 12% of the adult population (29.4 million), and the overall cost of diagnosing and managing OSA in the United States in 2015 was about $12.4 billion [34]. Fifty percent of these costs were spent on PAP and oral appliance treatment, 43% on surgical modifications of the upper airway, and about 7% on clinic visits and diagnostic tests. Moreover, that paper found that adults’ estimated economic burden of undiagnosed OSA was $149.6 billion [34].
The president of the KORL-HNS appointed the Task Force Chairman (YGJ) for this guideline, and the chairman recommended three experts as members of the guideline development group (GDG). Then, the process was approved by the board of directors of the KORL-HNS. Two members of the GDG were recommended and approved by the Korean Society of Sleep and Breathing. The GDG appointed nine sleep experts to review, correct, and conduct additional editing of the developed and written practice guideline, and they participated as co-authors in this clinical practice guideline. The GDG had complete editorial independence from the KORL-HNS. In developing this consensus-based clinical practice guideline, the GDG followed the Clinical Practice Guideline Development Manual, Third Edition, to create actionable statements [35]. The first meeting was held in July 2021, and there were 14 conference calls or meetings in total.
During a series of conference calls and meetings, the GDG defined the scope and objectives of the proposed guideline and selected key questions. The GDG determined that developing a consensus-based clinical practice guideline for nasal surgery would be most beneficial for clinicians who manage OSA patients. After deciding on the scope and objectives, this consensus-based clinical practice guideline was developed over 5 months. All key questions and corresponding action statements were created based on supporting evidence, balancing the benefits and potential harms. The recommendations contained in this consensus-based clinical practice guideline were developed based on the selected best literature published through December 2021, depending on the opinion of the GDG members. If we could not find enough data, we used a combination of clinical experience and expert consensus. The clinical practice guideline developed also underwent extensive external peer review. Finally, comments were compiled and reviewed by the GDG members. The final modified version of the clinical practice guideline was distributed and approved by the board of directors of the KORL-HNS. A total of 10 guideline recommendations regarding nasal surgery for OSA and PAP adherence are included, along with corresponding action statements and profiles present in the Results section.
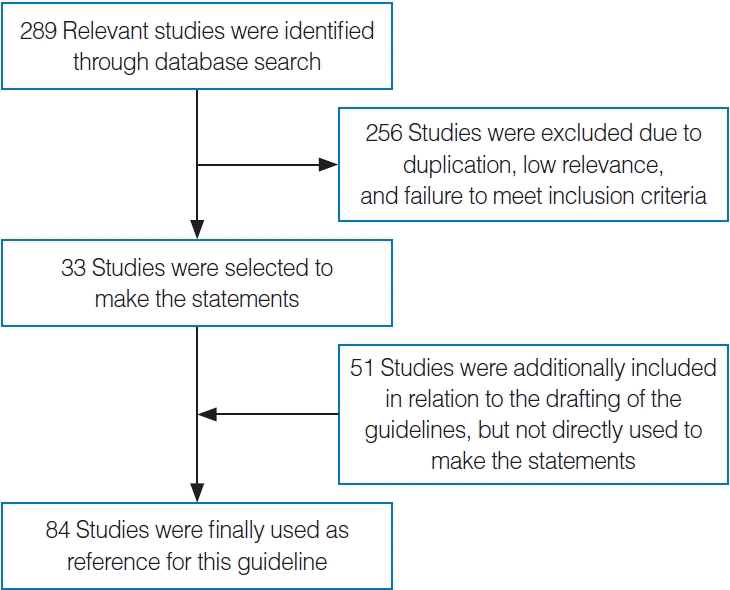
GDG conducted several literature searches from July to August 2021, using a validated filter strategy to identify clinical practice guidelines, systematic reviews, randomized controlled trials (RCTs), and related clinical studies. The following databases were searched for relevant studies: PubMed, Embase, and Google Scholar. The databases were searched with controlled vocabulary words and synonymous free text words relating to the topic of interest (effect of nasal surgery on OSA and compliance of continuous positive pressure). Types of nasal surgery include septoplasty, turbinate surgery, nasal valve surgery, septorhinoplasty, and endoscopic sinus surgery. The search was not limited to clinical study design or the English language. In certain instances, targeted searches for lower-level evidence were performed to address gaps in the systematic searches identified while writing the guideline. The investigation identified three clinical practice guidelines, 13 systematic reviews and meta-analyses, 11 RCTs, and 262 related studies. After removing duplicates and irrelevant records, the authors retained two RCTs and 31 case series (21 prospective cases and 10 retrospective ones) that met the inclusion criteria. An additional 51 related studies were identified that were related to the key action statements (Fig. 1).
A meta-analysis was performed on the indices to which several statements referred in order to clarify ambiguity (Supplementary Fig. 1, Supplementary Table 1). The mean and standard deviation (SD) of each index were collected and compared before and after surgery. The results were merged using statistical software (Comprehensive Meta-Analysis Version 2.0, Biostat). Only a Dersimonian-Laird random-effects model was used regardless of the degree of heterogeneity because we believed that the studies were essentially heterogeneous. Funnel plots and the Egger test were used to detect publication bias. When the funnel plot asymmetry was severe, or the one-tailed P-value in the Egger test was <0.05, the effect size was estimated after correcting the publication bias using the trim-and-fill method (Supplementary Fig. 2).
Guidelines are intended to produce optimal health outcomes for patients, minimize harm, and reduce inappropriate variations in clinical care [35]. The evidence-based approach to guideline development requires identifying, appraising, and summarizing the evidence supporting a policy and defining an explicit link between the evidence and statements [35]. Evidence-based statements reflect the quality of evidence and the balance of benefit and harm anticipated when the statement is followed. The definitions of terms used to describe evidence-based statements are listed in Tables 1 and 2 [35]. Guidelines are not intended to supersede professional judgment, but rather may be viewed as a relative constraint on individual clinicians’ discretion in particular clinical circumstances. Less frequent variation in practice is expected for a “strong recommendation” than what might be expected with only a “recommendation.” “Options” offer the most opportunity for practice variability (Table 3). Clinicians should always act and make decisions in a way that they believe will best serve their patients’ interests and needs, regardless of guideline recommendations. They must also operate within their scope of practice and according to their training. Guidelines represent the best judgment of a team of experienced clinicians and methodologists addressing the scientific evidence for a particular topic. Making recommendations about health practices involves value judgments on the desirability of various outcomes associated with management options. Values applied by the guideline panel sought to minimize harm and diminish unnecessary and inappropriate therapy. A primary goal of the panel was to be transparent and explicit about how values were applied and to document the process.
Levels of evidence
Aggregate grades of evidence by question type
Guideline definitions for evidence-based statements
The cost of developing this guideline was covered in full by the KORL-HNS. All conflicts are disclosed at the end of this document.
Each evidence-based statement is organized in a similar fashion: first, an evidence-based key action statement is presented in bold, followed by the strength of the recommendation in italics. Next, each key action statement is followed by the “action statement profile” with quality improvement opportunities, aggregate evidence quality, the level of confidence in the evidence, a benefit–harm assessment, and a statement of costs [35]. Additionally, there is an explicit statement of any value judgments, the role of patient preferences, clarification of any intentional vagueness by the panel, exclusions to the statement, any differences of opinion, and a repeated statement of the strength of the recommendation. Several paragraphs subsequently discuss the evidence base supporting the statement. In this guideline, shared decision-making refers to the exchange of information regarding treatment risks and benefits and the expression of patient preferences and values, which result in mutual responsibility in decisions regarding treatment and care. For an action statement where the evidence base demonstrates a clear benefit, clinicians should provide patients with clear and understandable information on the benefits to facilitate patient understanding and shared decision-making, leading to better patient adherence and outcomes. For statements where evidence is weaker or benefits are less certain, shared decision-making is beneficial, wherein a collaborative effort between the clinician and an informed patient makes the management decision. Factors related to patient preference include (but are not limited to) absolute benefits (number needed to treat), potential adverse effects (number needed to harm), the cost of drugs or procedures, and frequency and duration of treatment, as well as certain less-tangible factors such as religious and/or cultural beliefs or personal levels of desire for the intervention (Table 4).
Summary of evidence-based statements of nasal surgery on OSA
Statement 1. Nasal obstruction evaluation: the clinician should inquire about nasal obstruction and assess nasal patency during the evaluation of a patient with OSA.
A strong recommendation is made based on the consensus of the GDG, as well as a preponderance of benefits over harms.
• Quality improvement opportunity: To identify nasal obstruction, which is an exacerbating factor of OSA.
• Level of confidence in evidence: Low.
• Aggregate evidence quality: Grade X, based on exceptional situations where validating studies cannot be performed, and there is a clear preponderance of benefits over harms.
• Benefits: Identifying and reducing the exacerbating factors in OSA patients.
• Risks, harms, and costs: Minimal risk of evaluation and cost of evaluation procedures.
• Benefit–harm assessment: Preponderance of benefits over harms.
• Value judgment: None.
• Intentional vagueness: High-level evidence for the indications and effectiveness of nasal obstruction evaluation for OSA patients is still lacking.
• Role of patient preferences: Large.
• Exclusions: If there are no nasal obstruction symptoms or objective nasal lesions.
• Policy level: Strong recommendation.
• Differences of opinion: None.
Nasal obstruction is known to be a risk factor for OSA [20,36]. Therefore, OSA patients should be asked if they have nasal obstruction and should be evaluated. Nasal obstruction can be evaluated in various ways. Subjective evaluation methods include the visual analog scale (VAS) or the Nasal Obstruction Symptom Evaluation (NOSE) scale [37]. In addition to direct observation of the nasal cavity using an anterior rhinoscopy or an endoscope, objective measurements using instruments are sometimes performed [37]. Acoustic rhinometry can assess the cross-sectional area and volume of the nasal cavity, while rhinomanometry can measure nasal resistance [37]. Imaging equipment such as computed tomography or magnetic resonance imaging can be used to determine the overall structure and abnormalities of the nasal cavity [37].
Statement 2. Nasal obstruction: the clinician should consider referring the patient to an otorhinolaryngologist for the surgical treatment of nasal obstruction in osa patients with persistent symptoms despite appropriate medical therapy.
A strong recommendation is made based on highly consistent evidence, as well as a preponderance of benefits over harms.
• Quality improvement opportunity: To improve the subjective nasal obstruction and objective nasal patency of OSA patients.
• Level of confidence in evidence: High.
• Aggregate evidence quality: Grade C, based on observational studies.
• Benefits: Relieving nasal obstruction by widening the nasal passages.
• Risks, harms, and costs: Common complications from nasal surgery and the costs of hospitalization and the procedure.
• Benefit–harm assessment: Preponderance of benefits over harms.
• Value judgment: None.
• Intentional vagueness: High-level evidence for the indications and effectiveness of nasal surgery for OSA patients is still lacking.
• Role of patient preferences: Large.
• Exclusions: If there are no nasal obstruction symptoms or objective nasal lesions.
• Policy level: Strong recommendation.
• Differences of opinion: None.
Before describing this statement, it is necessary first to define medical therapy. The medical therapy mentioned in this clinical practice guideline refers to treatment to improve nasal obstruction, not treatments for OSA, such as CPAP. Medical therapy includes drugs such as oral medications, intravenous or intramuscular injections, and nasal sprays to control nasal symptoms in OSA patients, and PAP devices are not included in the category of medical therapy. For OSA patients, appropriate surgery can resolve nasal obstruction [38,39]. The types of surgery include septoplasty, turbinoplasty, endoscopic sinus surgery, and nasal valve surgery [40,41]. Most retrospective case series that measured subjective nasal obstruction using the VAS or NOSE scale reported that nasal obstruction improved after surgery [38,39, 42,43]. In addition, objective measures that can reflect nasal obstruction improved in all relevant studies after nasal surgery. The minimal cross-sectional area and nasal volume, as measured by acoustic rhinometry, showed an increase post-nasal surgery, while nasal resistance, as measured by rhinomanometry, demonstrated a decrease [36,38,44-48]. Some studies confirmed that the nasal cavity was enlarged according to anterior rhinoscopy or endoscopy after surgery [39]. Another study showed that the nasal valve volume and angle had increased using magnetic resonance imaging [49]. In addition, a computational fluid dynamics study comparing nasal resistance and airflow changes before and after septal surgery reported a 50% decrease in total nasal resistance after surgery compared to before surgery [50]. Based on these study results, nasal surgery has been shown to effectively address nasal obstruction in patients with OSA.
Statement 3. Quality of life or sleep quality: the clinician should consider referring the patient to an otorhinolaryngologist for the surgical treatment of nasal obstruction to improve sleep quality or quality of life in OSA patients with persistent nasal obstruction despite appropriate medical therapy.
A recommendation is made based on multiple systematic reviews, observational studies, and case series, as well as a preponderance of benefits over harms.
• Quality improvement opportunity:To inform clinicians that nasal surgery alone can effectively improve the sleep quality or quality of life of OSA patients with documented nasal airway obstruction.
• Level of confidence in evidence: Low.
• Aggregate evidence quality: Grade C, based on multiple systematic reviews, observational studies, and case series.
• Benefits: Improved quality of life and sleep quality, including facilitating less anxiety, fatigue, and reduced sleep latency or fragmentation.
• Risks, harms, and costs: Costs and risks of nasal surgery such as septoturbinoplasty or endoscopic sinus surgery.
• Benefit–harm assessment: Preponderance of benefits over harms.
• Value judgment:The GDG believes that nasal surgery needs to be more widely performed to improve quality of life and sleep quality.
• Intentional vagueness:There is still a lack of clear high-level evidence for how frequently nasal surgery should be done; the type and scope of surgery may also vary depending on the surgeon’s experience and medical judgment.
• Role of patient preferences: Low.
• Exclusions: Patients who are high-risk surgical and anesthetic candidates, have significant comorbidities, or do not want surgical treatment options.
• Policy level: Recommendation.
• Differences of opinion: None.
The supporting text of statement 3 is described together after statement 4.
Statement 4. Excessive daytime sleepiness: the clinician should consider referring the patient to an otorhinolaryngologist for the surgical treatment of nasal obstruction to address excessive daytime sleepiness in OSA patients with persistent symptoms despite appropriate medical therapy.
A recommendation is made based on multiple systematic reviews, meta-analyses, and well-designed clinical trials, as well as a preponderance of benefits over harms.
• Quality improvement opportunity:To inform clinicians that nasal surgery alone can effectively improve the excessive daytime sleepiness of OSA patients with documented nasal airway obstruction.
• Level of confidence in evidence: Medium.
• Aggregate evidence quality: Grade B, based on meta-analyses, systematic reviews, one RCT, and several case series.
• Benefits: Not only do the patient’s subjective nasal congestion symptoms improve, but their school, work, and safety issues related to daytime sleepiness are also reduced.
• Risks, harms, and costs: Costs and risks of nasal surgery such as septoturbinoplasty or endoscopic sinus surgery.
• Benefit–harm assessment: Preponderance of benefits over harms.
• Value judgment: None.
• Intentional vagueness:There is still a lack of clear high-level evidence for how frequently nasal surgery should be done; the type and scope of surgery may also vary depending on the surgeon’s experience and medical judgment.
• Role of patient preferences: Low.
• Exclusions: Patients who are high-risk surgical and anesthetic candidates, have significant comorbidities, or who do not want surgical treatment options.
• Policy level: Recommendation.
• Differences of opinion: None.
OSA is related to the repeated decrease or cessation of airflow, and whenever such an event occurs, it induces awakening during sleep, resulting in sleep fragmentation. OSA is thus associated with daytime sleepiness, fatigue, reduced sleep quality, and decreased work efficiency [51]. The Epworth Sleepiness Scale (ESS) is the most frequently used instrument to evaluate the subjective degree of daytime sleepiness. This scale has been applied in most studies to evaluate the subjective quality of life and sleep quality, and the Pittsburgh Sleep Quality Index (PSQI), 36-item Short-form Health Survey, the Symptom Checklist 90, and health-related quality of life have also been used. The GDG found 13 clinical studies [38,39,42,43,45,46,48,52-57] and six systematic reviews or meta-analyses [40,58-62] on the effects of nasal surgery on daytime sleepiness and quality of life in OSA patients. That research demonstrated that subjective symptoms such as ESS scores and quality of life significantly improved, which were very consistent results. The first meta-analysis of nasal surgery and sleep and quality of life was published in 2011 and analyzed the results of two controlled trials and 13 noncontrolled trials. After nasal surgery in OSA patients, the ESS significantly decreased from 10.6 to 7.1 points [58]. As mentioned above, quality of life was evaluated using various tools in several studies. Therefore, although it was possible to determine that quality of life tended to increase after surgery, it was not easy to conduct a systematic review or meta-analysis for each evaluation tool. In contrast, most studies evaluated daytime sleepiness using a single tool (the ESS), and our GDG performed a meta-analysis of reports using the ESS. A total of 16 controlled trials reporting ESS scores were included [63,64], and the analysis revealed significantly improved ESS scores in the nasal surgery group compared to the control group (standard difference in means, −1.091; 95% confidence interval [CI], −1.396 to 0.785; P<0.001) (Supplementary Fig. 1, Supplementary Table 1).
The results of this study’s meta-analysis are consistent with those of previously published studies. According to a meta-analysis conducted in 2019, ESS scores improved significantly in OSA patients after nasal surgery, but patients without OSA experienced no significant improvement in ESS [62]. In one study, an RCT was conducted in which a control group underwent sham surgery. The group that received nasal surgery showed significant improvements in ESS scores compared to the control group [48].
Statement 5. Snoring: the clinician may consider referring the patient to an otorhinolaryngologist for the surgical treatment of nasal obstruction to reduce snoring in OSA patients with persistent symptoms despite appropriate medical therapy.
An option is offered based on one systematic review, multiple observational studies, and case series, as well as a preponderance of benefits over harms.
• Quality improvement opportunity: To reduce snoring in OSA patients with nasal obstruction.
• Level of confidence in evidence: Low.
• Aggregate evidence quality: Grade C, based on one systematic review, multiple observational studies, and case series.
• Benefits: Reducing snoring.
• Risks, harms, and costs: There is a possibility of the usual complications from nasal surgery, and there may be perioperative risks due to OSA. Side effects of nasal surgery generally include nasal pain, infection, recurrence, and nasal bleeding, and specific adverse effects may differ according to the type of surgical procedure. The cost also depends on the type of surgical procedure and is relatively high.
• Benefit–harm assessment: Preponderance of benefits over harms.
• Value judgments: None.
• Intentional vagueness: The effect of isolated nasal surgery on snoring may vary depending on the type of surgical procedure; although nasal surgery alone is reported to improve subjective snoring in patients with OSA based on many studies, more clinical research is needed about whether objective snoring improves after nasal surgery.
• Role of patient preferences: None.
• Exclusions: When nasal surgery is contraindicated or when the patient refuses nasal surgery.
• Policy level: Option.
• Differences of opinion: None.
Snoring is a noisy respiratory sound during sleep caused by vibration of the upper airway’s soft tissue, including the nasal cavity, pharynx, and larynx. It is a kind of sleep-disordered breathing, such as OSA. Loud or frequent snoring can be an indicator or a warning sign of OSA. Snoring can be bothersome to other people and develop into OSA, leading to serious medical consequences [65]. Numerous studies have been conducted on the efficacy of isolated nasal surgery on subjective and objective snoring in OSA patients with nasal obstruction [38,56,58,66-71]. Most relevant clinical research has shown that nasal surgery significantly reduces snoring. Friedman et al. [66] reported that complete resolution of snoring was found in three patients (6%) and noted a decrease in snoring in 14 patients (28%) following nasal surgery. To estimate the efficacy of nasal surgery on snoring in OSA patients with nasal obstruction, Li et al. [38,69,70] performed three related clinical trials using questionnaires such as the Snore Outcomes Survey and Spouse/Bed Partner Survey. Their studies demonstrated that subjective snoring was significantly reduced after nasal surgery. Choi et al. [56] appraised the effectiveness of nasal surgery on objective snoring based on PSG data in OSA patients with nasal obstruction. They reported that snoring duration (snoring time/total sleep time×100) improved significantly from 32.2%±16.4% to 25.8%±18.6% following nasal surgery (P<0.05). Kim et al. [68] also investigated whether nasal surgery affected objective snoring and indicated that snoring duration declined from 44.1%±18.0% to 39.2%±17.5% after nasal surgery, albeit without statistical significance. Li et al. [58] reviewed data extracted from six papers related to the efficacy of nasal surgery on snoring based on VAS scores and individual questionnaires. They confirmed that snoring improved significantly following nasal surgery (P<0.05).
Statement 6. Respiratory disturbances (AHI/RDI): the clinician may consider referring the patient to an otorhinolaryngologist for the surgical treatment of nasal obstruction to improve respiratory disturbances during sleep in OSA patients with persistent symptoms despite appropriate medical therapy.
An option is offered based on multiple systematic reviews and RCTs, as well as a preponderance of benefits over harms.
• Quality improvement opportunity: To improve respiratory disturbances (AHI/RDI) for the treatment of patients with OSA; doing so improves the patient’s symptoms safely and is associated with a low financial burden.
• Level of confidence in evidence: Low.
• Aggregate evidence quality: Grade B, based on meta-analyses, RCTs, and multiple observational studies.
• Benefits: Reduction of significant respiratory disturbances.
• Risks, harms, and costs: There is a possibility of the usual complications from nasal surgery, and there may be perioperative risks due to OSA; the cost also depends on the hospitalization period and type of procedure.
• Benefit–harm assessment: Preponderance of benefits over harms.
• Value judgment: None.
• Intentional vagueness:There is still a lack of clear high-level evidence for how frequently or which type of nasal surgery should be done.
• Role of patient preferences: High.
• Exclusions: In cases where nasal breathing is not uncomfortable.
• Policy level: Option.
• Differences of opinion: Our meta-analysis of AHI values showed that nasal surgery significantly contributed to the improvement of AHI, but some other studies did not report significant statistical results.
It is difficult to accurately measure the effect of surgery because it is influenced by various patient, surgical, and environmental variables. PSG is the most common and easily accessible objective indicator to measure the effect of surgery in OSA patients. Among the PSG indices in the studies analyzed herein, indices of respiratory disturbances—such as AHI and the respiratory disturbance index (RDI), which are most widely used to evaluate the degree of sleep apnea—and indicators related to oxygen saturation during sleep were studied. The primary pathophysiology of OSA is based on structural obstruction of the upper airway. The closure of the nasal cavity, which is a component of the upper airway and is the first gateway through which external air enters, is likely to affect these respiratory indices [72].
Twenty-nine studies examined the AHI and/or RDI, which are the most widely used indicators to determine the severity of sleep apnea, including 25 clinical research studies and four meta-analyses [38-40,42-48,52,54-59,62,66,68,70,71,73-79]. In addition, the GDG performed a meta-analysis on respiratory disturbance with 23 controlled trials to evaluate the effect of nasal surgery on the AHI [38,39,42-48,52,54,56,57,68,70,71, 73-79]. The analysis showed a significantly decreased AHI in the nasal surgery group compared to the control group (standard difference in means, −0.252; 95% CI, −0.383 to −0.121; P<0.001) (Supplementary Fig. 1, Supplementary Table 1).
Previous meta-analysis reports also offered mixed results concerning the effectiveness of nasal surgery in improving the AHI [40,58,59,62]. As a result of our meta-analysis, where the largest number of studies were enrolled, nasal surgery significantly improved the AHI. Unfortunately, the RDI did not meet our analysis criteria and was not subjected to meta-analysis. Nonetheless, the findings of our meta-analysis helped to determine the recommendation level based on the results of previous meta-analyses and observational studies. Among the enrolled studies, the only report that conducted a meta-analysis of the RDI showed a significant decrease in the RDI after nasal surgery. Other reports also found a significant reduction in the RDI after nasal surgery [43,46,55,59,78,79].
Statement 7. Oxygen status (mean/minimum/oxygen desaturation index): the clinician may consider referring the patient to an otorhinolaryngologist for the surgical treatment of nasal obstruction to improve oxygen status during sleep in OSA patients with persistent symptoms despite appropriate medical therapy.
An option is offered based on RCTs and a preponderance of benefits over harms.
• Quality improvement opportunity: To improve the oxygen status during sleep for the treatment of patients with OSA; it improves the patient’s symptoms safely and is associated with a low financial burden.
• Level of confidence in evidence: Low.
• Aggregate evidence quality: Grade B, based on one RCT and multiple observational studies.
• Benefits: Improvement of oxygen status levels during sleep.
• Risks, harms, and costs: There is a possibility of the usual complications from nasal surgery, and there may be perioperative risks due to OSA; the cost also depends on the hospitalization period and the type of procedure.
• Benefit–harm assessment: Preponderance of benefits over harms.
• Value judgment: None.
• Intentional vagueness:There is still a lack of clear high-level evidence for how frequently or which type of nasal surgery should be done.
• Role of patient preferences: Low.
• Exclusions: In cases where nasal breathing is not uncomfortable.
• Policy level: Option.
• Differences of opinion: In our meta-analysis of the minimum o2 saturation results and several other analysis reports, nasal surgery was found to significantly contribute to the improvement of minimum o2 saturation, but some other studies did not report statistically significant results.
OSA is caused by upper airway obstruction, resulting in intermittent systemic hypoxia. This periodic occurrence of hypoxia is expressed in the form of oxygen saturation on PSG. Oxygen status during sleep is deeply involved in the pathophysiological complications of sleep apnea [80].
A total of 18 studies investigated the changes in oxygen status due to nasal surgery [38,42-48,52,56,57,66,70,71,73,74,78,79], including one RCT and 17 observational studies. In addition, the GDG performed a meta-analysis on oxygen status with 14 controlled trials for the effect of nasal surgery on minimum o2 saturation [38,42-47,52,56,57,70,73,74,79]. The analysis revealed a significantly improvement in the lowest o2 saturation in the nasal surgery group compared to the control group (standard difference in means, 0.261; 95% CI, 0.128–0.393; P<0.001) (Supplementary Fig. 1, Supplementary Table 1).
As in our meta-analysis and several previous reports, nasal surgery significantly improved minimum o2 saturation. Although the mean oxygen saturation and the oxygen desaturation index were not included in our meta-analysis, we determined our recommendation levels based on the results of several previous reports [43,47,48,52,57,71,74,79].
Statement 8. Optimal PAP level: the clinician should consider referring the patient to an otorhinolaryngologist for the surgical treatment of nasal obstruction to decrease the optimal PAP level in OSA patients with nasal obstruction related to poor PAP adherence if their symptoms persist despite appropriate medical therapy.
A recommendation is made based on one systematic review and meta-analysis, multiple observational studies, and case series, as well as a preponderance of benefits over harms.
• Quality improvement opportunity: To decrease the optimal pressure level of PAP in OSA patients with nasal obstruction related to poor PAP adherence and then ultimately improve PAP adherence.
• Level of confidence in evidence: Medium.
• Aggregate evidence quality: Grade B, based on one systematic review and meta-analysis, multiple observational studies, and case series.
• Benefits: Decreased optimal pressure level of PAP, which can facilitate improvements in PAP adherence.
• Risks, harms, and costs: There is a possibility of the usual complications from nasal surgery, and there may be perioperative risks due to OSA. The side effects of nasal surgery generally include nasal pain, infection, recurrence, and nasal bleeding, and specific adverse effects may differ according to the type of surgical procedure. The cost also depends on the type of surgical procedure and is relatively high.
• Benefit–harm assessment: Preponderance of benefits over harms.
• Value judgments: None.
• Intentional vagueness: None.
• Role of patient preferences: None.
• Exclusions: If the patient does not want surgery or is contraindicated for nasal surgery.
• Policy level: Recommendation.
• Differences of opinion: None.
PAP is the primary therapeutic method for OSA, and nasal obstruction is one of the common causes of poor PAP adherence [67]. In general, to improve nasal obstruction, conservative or medical management is first tried; then, if there is no improvement, surgical treatment should be considered. Multiple prospective and retrospective studies have been conducted on the effect of nasal surgery alone on PAP therapy, including optimal pressure levels in OSA patients with nasal obstruction [39,44, 66,71,81-83]. Numerous studies have shown that the mean optimal PAP level decreased considerably after nasal surgery, and most related studies reported that nasal surgery significantly reduced the optimal pressure level of PAP. Friedman et al. [66] investigated whether nasal surgery changed the optimal pressure level of PAP in OSA patients and found that the optimal PAP level decreased significantly from 9.3 to 6.7 cm H2O after nasal surgery (P<0.01). Nakata et al. [44] also evaluated the effectiveness of nasal surgery on the optimal pressure level of PAP in PAP-intolerant patients with severe OSA and nasal obstruction and showed that the optimal PAP level was reduced significantly from 16.8±1.1 to 12.0±1.9 cm H2O (P<0.05). Sufioglu et al. [71] tried to determine whether nasal surgery influenced the optimal PAP level in OSA patients with nasal obstruction and reported that the optimal PAP level was diminished from 11.2± 1.2 to 10.4±1.4 cm H2O following nasal surgery, albeit without statistical significance (P=0.062). Camacho et al. [17] performed a systemic literature review and meta-analysis based on merged data from seven clinical trials associated with optimal PAP levels to demonstrate the efficacy of nasal surgery on optimal PAP levels. As a result, the optimal pressure level of PAP decreased from 11.6±2.2 to 9.5±2.0 cm H2O (mean±SD), and the pooled random-effects analysis demonstrated a statistically significant decrease in the average optimal pressure, with a mean difference of −2.66 cm H2O (95% CI, −3.65 to −1.67 cm H2O; overall effect, Z score=5.27; P<0.001).
Statement 9. PAP usage time: the clinician may consider referring the patient to an otorhinolaryngologist for the surgical treatment of nasal obstruction to increase PAP usage time in OSA patients with nasal obstruction related to poor PAP adherence if their symptoms persist despite appropriate medical therapy.
An option is offered based on one systematic review, multiple observational studies, case series, and a preponderance of benefits over harms.
• Quality improvement opportunity: To increase the usage time of PAP in OSA patients with nasal obstruction related to poor PAP adherence and then ultimately improve PAP adherence.
• Level of confidence in evidence: Low.
• Aggregate evidence quality: Grade C, based on one systematic review, multiple observational studies, and case series.
• Benefits: Increased usage time of PAP, which can affect the improvement of PAP adherence.
• Risks, harms, and costs: There is a possibility of the usual complications from nasal surgery, and there may be perioperative risks due to OSA. Side effects of nasal surgery generally include nasal pain, infection, recurrence, and nasal bleeding, and specific adverse effects may differ according to the type of surgical procedure. The cost also depends on the type of surgical procedure and is relatively high.
• Benefit–harm assessment: Preponderance of benefits over harms.
• Value judgments: None.
• Intentional vagueness: The effect of nasal surgery alone on increased PAP usage time may vary depending on the type of surgical procedure. Most related studies have shown that isolated nasal surgery increases PAP usage time in patients with OSA. However, clinical investigations are still insufficient, and further studies are required to confirm these findings.
• Role of patient preferences: None.
• Exclusions: If the patient does not want surgery or is contraindicated for nasal surgery.
• Policy level: Option.
• Differences of opinion: None.
In OSA patients with PAP therapy, nasal obstruction can be associated with various reasons for poor PAP adherence, including difficulty in nasal breathing, mouth leaks, and unintentional mask removal [67]. Therefore, surgical corrections for obstructive nasal anatomy can play an essential role in improving PAP compliance in OSA patients with nasal obstruction related to PAP non-adherence. Poirier et al. [81] examined the efficacy of isolated nasal surgery on PAP management, focusing on parameters such as PAP usage time and optimal level, in 18 non-compliant PAP patients with OSA. Their study established that the PAP usage time increased significantly from 0.5 to 5.0 hr/day (P<0.05), and the optimal PAP level decreased significantly from 11.9 to 8.2 cm H2O (P<0.05) after nasal surgery when the data were re-analyzed without two patients whose conditions normalized with surgical therapy. Park et al. [39] also evaluated the impact of nasal surgery alone on PAP adherence in OSA patients with nasal obstruction. They reported that all seven PAPintolerant patients could adhere to PAP treatment, based on the commonly accepted definition of adequate compliance, following nasal surgery. To evaluate the therapeutic effect of nasal surgery (radiofrequency reduction of inferior turbinate hypertrophy) in OSA patients with PAP treatment, Powell et al. [84] carried out a double-blind, randomized placebo-controlled trial and found that the PAP usage time of the intervention group was 32 minutes longer than that of the control group, but without statistical significance. Camacho et al. [17] reviewed 11 clinical articles (153 patients) related to the effect of nasal surgery on PAP therapy, including PAP usage time, tolerance, and adherence. They concluded that overall outcomes of PAP use, acceptance, tolerance, and adherence improved after nasal surgery. Additionally, PAP usage time increased from a mean±SD of 3.0±3.1 to 5.5±2.0 hr/day in OSA patients with nasal obstruction related to poor PAP adherence.
Statement 10. Re-evaluation of the efficacy of nasal surgery: the clinician should reassess the efficacy of nasal surgery, including nasal patency and sleep status, using both subjective and objective methods in OSA patients when their recovery is deemed complete, with re-evaluation intervals determined at the discretion of the clinician.
A recommendation is made based on multiple systematic reviews, meta-analyses, and well-designed clinical trials, as well as a preponderance of benefits over harms.
• Quality improvement opportunity:To reinforce the need for an appropriate assessment of the effect of nasal surgery alone in OSA patients and to improve long-term patient management by the clinician based on the patient’s postoperative status as reassessed with subjective and objective measurements.
• Level of confidence in evidence: Low.
• Aggregate evidence quality: Grade X, based on experts’ opinions.
• Benefits: Increasing patient satisfaction by individually managing the patient after postoperative re-evaluations.
• Risks, harms, and costs:There are no risks or harms; however, there may be costs associated with re-evaluations, including PSG.
• Benefit–harm assessment: Preponderance of benefits over harms.
• Value judgment: None.
• Intentional vagueness: None.
• Role of patient preferences: Low.
• Exclusions: None.
• Policy level: Recommendation.
• Differences of opinion: None.
After surgical treatment for OSA, it is essential to evaluate the patient’s sleep state and compare it to their sleep before surgery. In the case of multi-level surgery, the clinician should re-prescribe a PSG test 2–3 months after surgery or when the patient’s recovery is considered complete and compare the results to the preoperative results. However, for nasal surgery performed alone for OSA, there was no clinical guideline on whether a postoperative examination should be performed or what type of examination should be used to evaluate it. Therefore, the GDG prepared this statement based on the evidence of statements 2 and 3, which describe the effect of nasal surgery alone on the patient’s subjective and objective outcomes.
The primary purpose of nasal surgery in OSA patients is to improve their nasal symptoms, with the improvement of sleeprelated factors being an additional benefit. Therefore, if significant apnea or hypopnea remains after nasal surgery, conventional OSA treatment such as CPAP and multi-level surgery should be considered.
Subjective measures such as the ESS, PSQI, NOSE scale, and VAS (e.g., nasal obstruction, snoring, etc.) consistently improved consistently in multiple studies of patients who received nasal surgery alone for OSA. These questionnaires can quantify the patient’s subjective symptoms, which can be challenging to evaluate and help clearly explain the surgical outcome. In addition, the results of verified questionnaires conducted before and after surgery can be used as valuable data for future research on sleep apnea.
Many clinical trials have assessed objective measures, such as rhinomanometry, acoustic rhinometry, and PSG, in patients with OSA after isolated nasal surgery. Most studies have shown significant changes in nasal resistance using rhinomanometry and the minimal cross-sectional area using acoustic rhinometry. However, the postoperative outcomes of studies using PSG as an evaluation method tended to be heterogeneous. Some studies have reported significant improvements in objective parameters, such as sleep efficiency, sleep architecture, arousal index, respiratory disturbances, and oxygen status after nasal surgery alone, while others have not found statistically significant results. Therefore, it is crucial to evaluate patients’ objective sleep status using PSG.
Many clinical trials have published reports on nasal surgery for OSA, but the evidence level of each clinical study is often not high. Additionally, most studies are retrospective investigations, and the types and scope of nasal surgery applied in these studies are very diverse. For example, a RCT study used sham surgery in 2008 [48], but recently, there have been many ethical issues with conducting controlled trials for surgery.
A fundamental problem in interpreting several existing studies is that a clear standard definition of the indications for surgery does not yet exist. In addition, there are no formal criteria for an optimal nasal cross-sectional area or nasal resistance for having normal sleep without nasal obstruction. Thus, there is confusion in determining the extent of surgery and selecting surgical candidates. To solve this problem, it is worth analyzing the degree of change in the nasal structure before and after surgery using an objective method such as computational fluid dynamics and analyzing these changes alongside sleep parameters.
Despite these limitations, the GDG attempted to analyze the effect of nasal surgery on subjective and objective indicators of OSA and to derive reasonable, non-skewed results through statistical methods and consensus. As a result, the GDG reached the following conclusions: isolated nasal surgery in OSA patients improved subjective parameters, such as quality of life/sleep quality, ESS, and snoring, and improved objective indicators, such as respiratory disturbances, oxygen status, optimal PAP level, and PAP usage time. However, regarding quality of life, which is essential in recent real-world data, various evaluation tools were used in several studies, so it was impossible to standardize and analyze each result. Therefore, it will be necessary to develop and disseminate a standardized tool that can be applied after therapeutic attempts for OSA.
When septal surgery is performed in OSA patients, normal nasal breathing becomes difficult immediately after surgery due to nasal packing, crust, or swelling. Therefore, it is generally challenging for patients who use CPAP as a nasal mask before surgery to use CPAP for a certain period immediately after surgery. In such cases, the patient changes to an oronasal mask or temporarily stops using CPAP. This GDG tried to recommend this as a separate statement but did not find sufficient evidence. However, it is hoped that further research on this topic will be conducted in the future.
The GDG, supported by the KORL-HNS, did not attempt to provide guidance on all the effects of nasal surgery on OSA. In addition, this guideline cannot be applied to all OSA patients, as the management of OSA is very diverse depending on the treatment area and propensity of the healthcare provider treating OSA patients, their conditions, and their preferences.
Instead, we have attempted to help care providers by providing evidence-based information on areas that may be particularly confusing about recommending nasal surgery to OSA patients or referring patients to an otorhinolaryngologist for nasal surgery. However, as medical knowledge and technology are continually evolving and expanding, this guideline may be revised and new sections added to it in the future. Clinicians can also apply their own techniques based on their clinical judgment and evidence that this guideline may not include. Such methods may be included in future guidelines if scientifically verified. In addition, the GDG emphasizes that this practice guideline does not contain information on all forms of care and treatment decisions. Thus, some efficacious treatment methods may not be included in this practice guideline.
▪ The Korean Society of Otorhinolaryngology-Head and Neck Surgery and the Korean Society of Sleep and Breathing developed a clinical practice guideline for the use of nasal surgery to treat obstructive sleep apnea (OSA).
▪ This guideline is intended for all healthcare professionals treating OSA patients, regardless of their specialty, and discusses the objective and subjective effects of nasal surgery on patients’ sleep quality and the use of positive airway pressure (PAP) devices.
▪ If OSA patients have nasal obstruction, an evaluation to determine the cause of the nasal obstruction is necessary.
▪ For patients with OSA whose nasal obstruction does not improve with appropriate medical therapy, nasal surgery such as septoturbinoplasty or endoscopic sinus surgery should be considered as a way to improve sleep quality, reduce excessive daytime sleepiness, and improve objective sleep and PAP device-related parameters.
ACKNOWLEDGMENTS
The authors express their deep gratitude to Professor Se Hun Kim, the chairman of the Korean Society of Otorhinolaryngology–Head and Neck Surgery, and Professor Jeong Hun Kim, the president of the Korean Society of Sleep and Breathing, for their full support in the writing of this clinical practice guideline.
Supplementary materials
Supplementary materials can be found online at https://doi.org/10.21053/ceo.2022.01361.
Supplementary Fig. 1.
Forest plot for Epworth Sleepiness Scale (ESS), apnea-hypopnea index (AHI), and lowest o2 saturation. After nasal surgery, ESS and AHI decreased significantly, and the lowest o2 saturation increased significantly. Std diff, standardized difference; CI, confidence interval.
Supplementary Fig. 2.
Funnel plots of the studies including the Epworth Sleepiness Scale (ESS), apnea-hypopnea index (AHI), and the lowest O2 saturation. For ESS, the scatterplot is asymmetric, while for AHI and lowest o2 saturation, it is symmetric.
REFERENCES
1. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002; May. 165(9):1217–39.
2. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study Cohort. Sleep. 2016; Jul. 39(7):1353–9.
3. Aronson D, Nakhleh M, Zeidan-Shwiri T, Mutlak M, Lavie P, Lavie L. Clinical implications of sleep disordered breathing in acute myocardial infarction. PLoS One. 2014; Feb. 9(2):e88878.
4. Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019; Apr. 40(14):1149–57.
5. Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012; Jul. 186(2):190–4.
6. Veasey SC, Rosen IM. Obstructive sleep apnea in adults. N Engl J Med. 2019; Apr. 380(15):1442–9.
7. Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011; Aug. 306(6):613–9.
8. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MS, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019; Aug. 7(8):687–98.
9. Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014; Apr. 10(4):355–62.
10. Gurubhagavatula I, Patil S, Meoli A, Olson R, Sullivan S, Berneking M, et al. Sleep apnea evaluation of commercial motor vehicle operators. J Clin Sleep Med. 2015; Mar. 12(3):285–6.
11. Kales SN, Czeisler CA. Obstructive sleep apnea and work accidents: time for action. Sleep. 2016; Jun. 39(6):1171–3.
12. Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009; Jun. 5(3):263–76.
13. Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006; Mar. 29(3):375–80.
14. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008; Feb. 5(2):173–8.
15. Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007; Jun. 30(6):711–9.
16. Sunwoo BY, Light M, Malhotra A. Strategies to augment adherence in the management of sleep-disordered breathing. Respirology. 2020; Apr. 25(4):363–71.
17. Camacho M, Riaz M, Capasso R, Ruoff CM, Guilleminault C, Kushida CA, et al. The effect of nasal surgery on continuous positive airway pressure device use and therapeutic treatment pressures: a systematic review and meta-analysis. Sleep. 2015; Feb. 38(2):279–86.
18. Wilhelm CP, deShazo RD, Tamanna S, Ullah MI, Skipworth LB. The nose, upper airway, and obstructive sleep apnea. Ann Allergy Asthma Immunol. 2015; Aug. 115(2):96–102.
19. Friedman M, Maley A, Kelley K, Leesman C, Patel A, Pulver T, et al. Impact of nasal obstruction on obstructive sleep apnea. Otolaryngol Head Neck Surg. 2011; Jun. 144(6):1000–4.
20. Young T, Finn L, Kim H; The University of Wisconsin Sleep and Respiratory Research Group. Nasal obstruction as a risk factor for sleep-disordered breathing. J Allergy Clin Immunol. 1997; Feb. 99(2):S757–62.
21. Lofaso F, Coste A, d’Ortho MP, Zerah-Lancner F, Delclaux C, Goldenberg F, et al. Nasal obstruction as a risk factor for sleep apnoea syndrome. Eur Respir J. 2000; Oct. 16(4):639–43.
22. Ryan CM, Bradley TD. Pathogenesis of obstructive sleep apnea. J Appl Physiol (1985). 2005; Dec. 99(6):2440–50.
23. Ryan CF. Sleep • 9: an approach to treatment of obstructive sleep apnoea/hypopnoea syndrome including upper airway surgery. Thorax. 2005; Jul. 60(7):595–604.
24. Fitzpatrick MF, McLean H, Urton AM, Tan A, O’Donnell D, Driver HS. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur Respir J. 2003; Nov. 22(5):827–32.
25. Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012; Jan. 156(2):115–22.
26. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993; Apr. 328(17):1230–5.
27. Kim J, In K, Kim J, You S, Kang K, Shim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004; Nov. 170(10):1108–13.
28. Knauert M, Naik S, Gillespie MB, Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J Otorhinolaryngol Head Neck Surg. 2015; Sep. 1(1):17–27.
29. Albarrak M, Banno K, Sabbagh AA, Delaive K, Walld R, Manfreda J, et al. Utilization of healthcare resources in obstructive sleep apnea syndrome: a 5-year follow-up study in men using CPAP. Sleep. 2005; Oct. 28(10):1306–11.
30. Berger MB, Sullivan W, Owen R, Wu C. A corporate driven sleep apnea detection and treatment program: results and challenges [Internet]. Precision Pulmonary Diagnostics; 2005 [cited 2023 Mar 16]. Available from: https://www.precisionpulmonary.com/articles/corporate_program/.
31. Hoffman B, Wingenbach DD, Kagey AN, Schaneman JL, Kasper D. The long-term health plan and disability cost benefit of obstructive sleep apnea treatment in a commercial motor vehicle driver population. J Occup Environ Med. 2010; May. 52(5):473–7.
32. Kapur V, Blough DK, Sandblom RE, Hert R, de Maine JB, Sullivan SD, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999; Sep. 22(6):749–55.
33. Tarasiuk A, Greenberg-Dotan S, Brin YS, Simon T, Tal A, Reuveni H. Determinants affecting health-care utilization in obstructive sleep apnea syndrome patients. Chest. 2005; Sep. 128(3):1310–4.
34. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. Frost & Sullivan;2016.
35. Rosenfeld RM, Shiffman RN, Robertson P; Department of Otolaryngology State University of New York Downstate. Clinical practice guideline development manual, third edition: a quality-driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2013; Jan. 148(1 Suppl):S1–55.
36. Morris LG, Burschtin O, Lebowitz RA, Jacobs JB, Lee KC. Nasal obstruction and sleep-disordered breathing: a study using acoustic rhinometry. Am J Rhinol. 2005; Jan-Feb. 19(1):33–9.
37. Rimmer J, Hellings P, Lund VJ, Alobid I, Beale T, Dassi C, et al. European position paper on diagnostic tools in rhinology. Rhinology. 2019; Jul. 57(Suppl 28):1–41.
38. Li HY, Lin Y, Chen NH, Lee LA, Fang TJ, Wang PC. Improvement in quality of life after nasal surgery alone for patients with obstructive sleep apnea and nasal obstruction. Arch Otolaryngol Head Neck Surg. 2008; Apr. 134(4):429–33.
39. Park CY, Hong JH, Lee JH, Lee KE, Cho HS, Lim SJ, et al. Clinical effect of surgical correction for nasal pathology on the treatment of obstructive sleep apnea syndrome. PLoS One. 2014; Jun. 9(6):e98765.
40. Wu J, Zhao G, Li Y, Zang H, Wang T, Wang D, et al. Apnea-hypopnea index decreased significantly after nasal surgery for obstructive sleep apnea: a meta-analysis. Medicine (Baltimore). 2017; Feb. 96(5):e6008.
41. Mickelson SA. Nasal surgery for obstructive sleep apnea syndrome. Otolaryngol Clin North Am. 2016; Dec. 49(6):1373–81.
42. Xiao Y, Han D, Zang H, Wang D. The effectiveness of nasal surgery on psychological symptoms in patients with obstructive sleep apnea and nasal obstruction. Acta Otolaryngol. 2016; Jun. 136(6):626–32.
43. Kim SD, Jung DW, Lee JW, Park JH, Mun SJ, Cho KS. Relationship between allergic rhinitis and nasal surgery success in patients with obstructive sleep apnea. Am J Otolaryngol. 2021; Nov-Dec. 42(6):103079.
44. Nakata S, Noda A, Yagi H, Yanagi E, Mimura T, Okada T, et al. Nasal resistance for determinant factor of nasal surgery in CPAP failure patients with obstructive sleep apnea syndrome. Rhinology. 2005; Dec. 43(4):296–9.
45. Nakata S, Noda A, Yasuma F, Morinaga M, Sugiura M, Katayama N, et al. Effects of nasal surgery on sleep quality in obstructive sleep apnea syndrome with nasal obstruction. Am J Rhinol. 2008; Jan-Feb. 22(1):59–63.
46. Uz U, Gunhan K, Yilmaz H, Unlu H. The evaluation of pattern and quality of sleep in patients with chronic rhinosinusitis with nasal polyps. Auris Nasus Larynx. 2017; Dec. 44(6):708–12.
47. Tagaya M, Otake H, Suzuki K, Yasuma F, Yamamoto H, Noda A, et al. The comparison of nasal surgery and CPAP on daytime sleepiness in patients with OSAS. Rhinology. 2017; Sep. 55(3):269–73.
48. Koutsourelakis I, Georgoulopoulos G, Perraki E, Vagiakis E, Roussos C, Zakynthinos SG. Randomised trial of nasal surgery for fixed nasal obstruction in obstructive sleep apnoea. Eur Respir J. 2008; Jan. 31(1):110–7.
49. Bican A, Kahraman A, Bora I, Kahveci R, Hakyemez B. What is the efficacy of nasal surgery in patients with obstructive sleep apnea syndrome? J Craniofac Surg. 2010; Nov. 21(6):1801–6.
50. Na Y, Kim YJ, Kim HY, Jung YG. Improvements in airflow characteristics and effect on the NOSE score after septoturbinoplasty: a computational fluid dynamics analysis. PLoS One. 2022; Nov. 17(11):e0277712.
51. Cai Y, Goldberg AN, Chang JL. The nose and nasal breathing in sleep apnea. Otolaryngol Clin North Am. 2020; Jun. 53(3):385–95.
52. Tosun F, Kemikli K, Yetkin S, Ozgen F, Durmaz A, Gerek M. Impact of endoscopic sinus surgery on sleep quality in patients with chronic nasal obstruction due to nasal polyposis. J Craniofac Surg. 2009; Mar. 20(2):446–9.
53. Tajudeen BA, Brooks SG, Yan CH, Kuan EC, Schwartz JS, Suh JD, et al. Quality-of-life improvement after endoscopic sinus surgery in patients with obstructive sleep apnea. Allergy Rhinol (Providence). 2017; Mar. 8(1):25–31.
54. Moxness MH, Nordgard S. An observational cohort study of the effects of septoplasty with or without inferior turbinate reduction in patients with obstructive sleep apnea. BMC Ear Nose Throat Disord. 2014; Oct. 14:11.
55. Hisamatsu K, Kudo I, Makiyama K. The effect of compound nasal surgery on obstructive sleep apnea syndrome. Am J Rhinol Allergy. 2015; Nov-Dec. 29(6):e192–6.
56. Choi JH, Kim EJ, Kim YS, Kim TH, Choi J, Kwon SY, et al. Effectiveness of nasal surgery alone on sleep quality, architecture, position, and sleep-disordered breathing in obstructive sleep apnea syndrome with nasal obstruction. Am J Rhinol Allergy. 2011; Sep-Oct. 25(5):338–41.
57. Nourizadeh N, Rasoulian B, Majidi MR, Ardani AR, Rezaeitalab F, Asadpour H. Sleep quality after endoscopic sinus surgery in patients with sinonasal polyposis. Auris Nasus Larynx. 2019; Dec. 46(6):866–70.
58. Li HY, Wang PC, Chen YP, Lee LA, Fang TJ, Lin HC. Critical appraisal and meta-analysis of nasal surgery for obstructive sleep apnea. Am J Rhinol Allergy. 2011; Jan-Feb. 25(1):45–9.
59. Ishii L, Roxbury C, Godoy A, Ishman S, Ishii M. Does nasal surgery improve OSA in patients with nasal obstruction and OSA?: a meta-analysis. Otolaryngol Head Neck Surg. 2015; Sep. 153(3):326–33.
60. Johnson DM, Soose RJ. Updated nasal surgery for obstructive sleep apnea. Adv Otorhinolaryngol. 2017; 80:66–73.
61. Guttemberg MD, Mata FA, Nakanishi M, de Andrade KR, Pereira MG. Sleep quality assessment in chronic rhinosinusitis patients submitted to endoscopic sinus surgery: a meta-analysis. Braz J Otorhinolaryngol. 2019; Nov-Dec. 85(6):780–7.
62. Wang M, Liu SY, Zhou B, Li Y, Cui S, Huang Q. Effect of nasal and sinus surgery in patients with and without obstructive sleep apnea. Acta Otolaryngol. 2019; May. 139(5):467–72.
63. Victores AJ, Takashima M. Effects of nasal surgery on the upper airway: a drug-induced sleep endoscopy study. Laryngoscope. 2012; Nov. 122(11):2606–10.
64. Virkkula P, Hytonen M, Bachour A, Malmberg H, Hurmerinta K, Salmi T, et al. Smoking and improvement after nasal surgery in snoring men. Am J Rhinol. 2007; Mar-Apr. 21(2):169–73.
65. Deary V, Ellis JG, Wilson JA, Coulter C, Barclay NL. Simple snoring: not quite so simple after all? Sleep Med Rev. 2014; Dec. 18(6):453–62.
66. Friedman M, Tanyeri H, Lim JW, Landsberg R, Vaidyanathan K, Caldarelli D. Effect of improved nasal breathing on obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000; Jan. 122(1):71–4.
67. Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest. 2007; Sep. 132(3):1057–72.
68. Kim ST, Choi JH, Jeon HG, Cha HE, Kim DY, Chung YS. Polysomnographic effects of nasal surgery for snoring and obstructive sleep apnea. Acta Otolaryngol. 2004; Apr. 124(3):297–300.
69. Li HY, Lee LA, Wang PC, Chen NH, Lin Y, Fang TJ. Nasal surgery for snoring in patients with obstructive sleep apnea. Laryngoscope. 2008; Feb. 118(2):354–9.
70. Li HY, Lee LA, Wang PC, Fang TJ, Chen NH. Can nasal surgery improve obstructive sleep apnea: subjective or objective? Am J Rhinol Allergy. 2009; Nov-Dec. 23(6):e51–5.
71. Sufioglu M, Ozmen OA, Kasapoglu F, Demir UL, Ursavas A, Erisen L, et al. The efficacy of nasal surgery in obstructive sleep apnea syndrome: a prospective clinical study. Eur Arch Otorhinolaryngol. 2012; Feb. 269(2):487–94.
72. Park DY, Kim JS, Park B, Kim HJ. Risk factors and clinical prediction formula for the evaluation of obstructive sleep apnea in Asian adults. PLoS One. 2021; Feb. 16(2):e0246399.
73. Iwata N, Nakata S, Inada H, Kimura A, Hirata M, Yasuma F. Clinical indication of nasal surgery for the CPAP intolerance in obstructive sleep apnea with nasal obstruction. Auris Nasus Larynx. 2020; Dec. 47(6):1018–22.
74. Yalamanchali S, Cipta S, Waxman J, Pott T, Joseph N, Friedman M. Effects of endoscopic sinus surgery and nasal surgery in patients with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2014; Jul. 151(1):171–5.
75. Series F, St Pierre S, Carrier G. Effects of surgical correction of nasal obstruction in the treatment of obstructive sleep apnea. Am Rev Respir Dis. 1992; Nov. 146(5 Pt 1):1261–5.
76. Verse T, Maurer JT, Pirsig W. Effect of nasal surgery on sleep-related breathing disorders. Laryngoscope. 2002; Jan. 112(1):64–8.
77. Virkkula P, Bachour A, Hytonen M, Salmi T, Malmberg H, Hurmerinta K, et al. Snoring is not relieved by nasal surgery despite improvement in nasal resistance. Chest. 2006; Jan. 129(1):81–7.
78. Shuaib SW, Undavia S, Lin J, Johnson CM Jr, Stupak HD. Can functional septorhinoplasty independently treat obstructive sleep apnea? Plast Reconstr Surg. 2015; Jun. 135(6):1554–65.
79. Mandour YM, Abo Youssef SM, Moussa HH. Polysomnographic and pulmonary function changes in patients with sleep problems after septoplasty with turbinectomy. Am J Otolaryngol. 2019; Mar-Apr. 40(2):187–90.
80. In SM, Park DY, Lee KI, Gu G, Kim HJ. The effects of intermittent hypoxia on human nasal mucosa. Sleep Breath. 2021; Sep. 25(3):1453–60.
81. Poirier J, George C, Rotenberg B. The effect of nasal surgery on nasal continuous positive airway pressure compliance. Laryngoscope. 2014; Jan. 124(1):317–9.
82. Reilly EK, Boon MS, Vimawala S, Chitguppi C, Patel J, Murphy K, et al. Tolerance of continuous positive airway pressure after sinonasal surgery. Laryngoscope. 2021; Mar. 131(3):E1013–8.
83. Zonato AI, Bittencourt LR, Martinho FL, Gregorio LC, Tufik S. Upper airway surgery: the effect on nasal continuous positive airway pressure titration on obstructive sleep apnea patients. Eur Arch Otorhinolaryngol. 2006; May. 263(5):481–6.
84. Powell NB, Zonato AI, Weaver EM, Li K, Troell R, Riley RW, et al. Radiofrequency treatment of turbinate hypertrophy in subjects using continuous positive airway pressure: a randomized, double-blind, placebo-controlled clinical pilot trial. Laryngoscope. 2001; Oct. 111(10):1783–90.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download