Abstract
References
Fig. 1
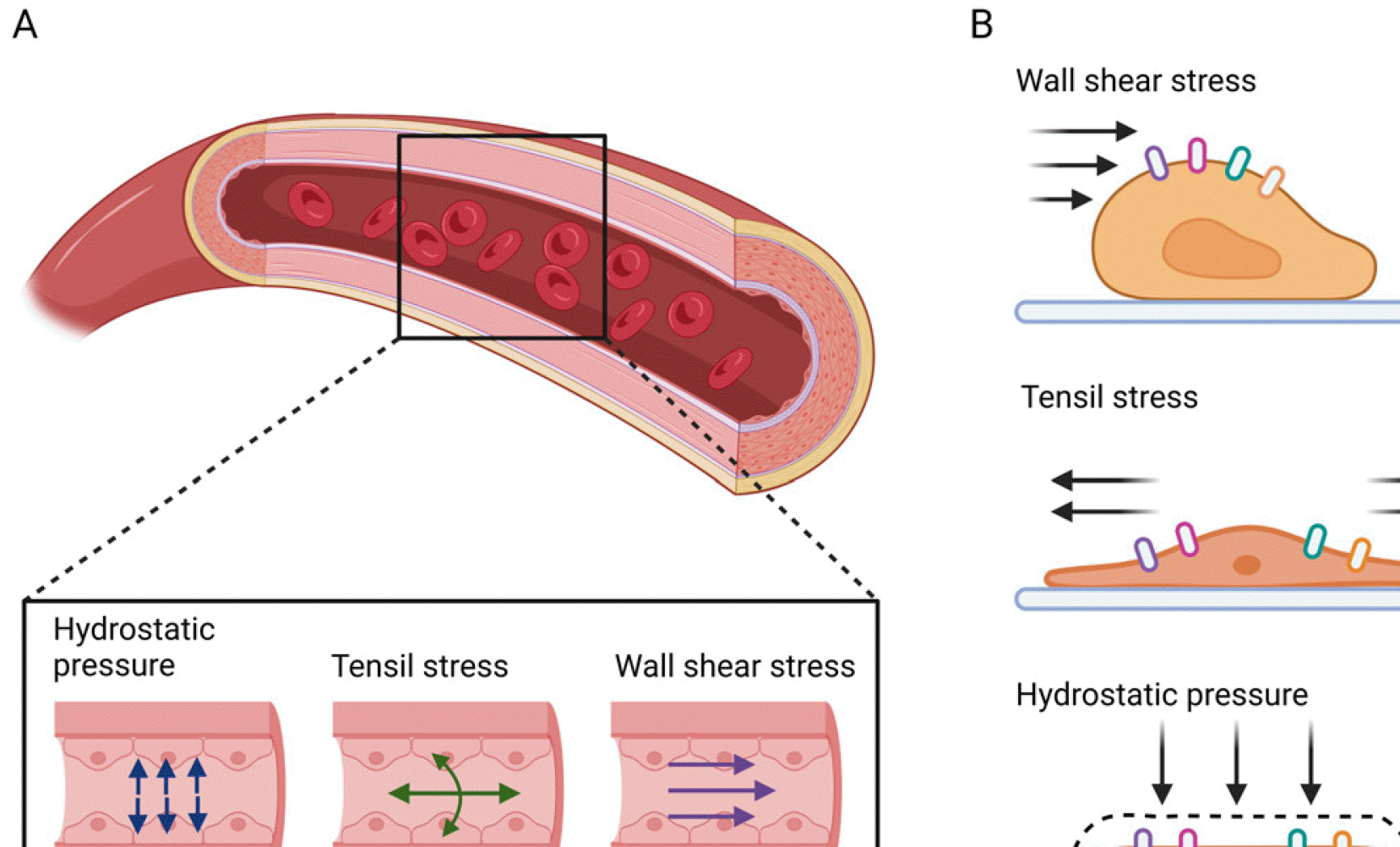
Fig. 2
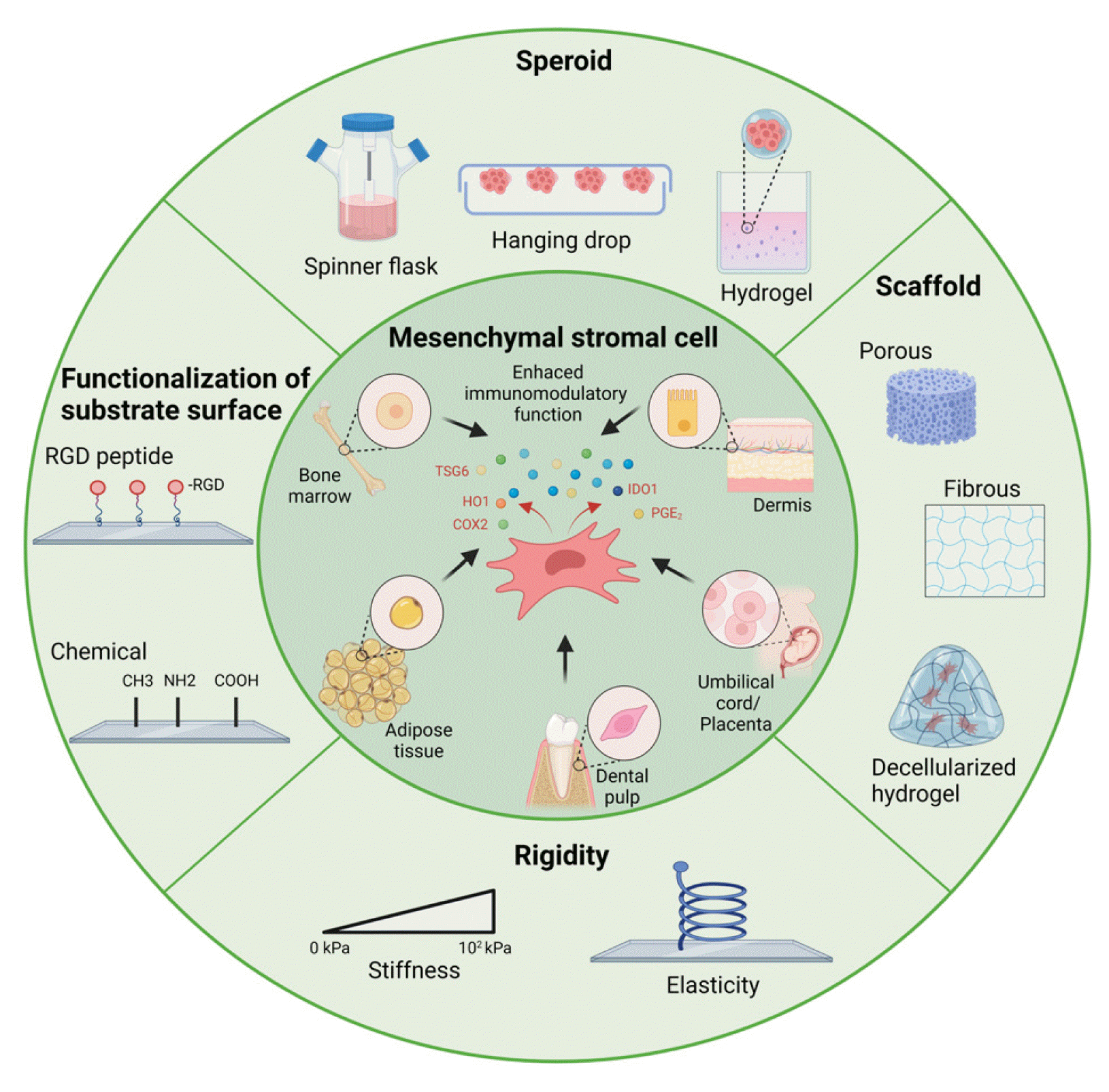
Table 1
| Biomechanical force | MSC origin | Bioreactor | Immunomodulation | References |
|---|---|---|---|---|
| Shear stress | Human | Microfluidics devices | Upregulated anti-inflammatory mediators (COX-2, HO-1, PGE2) and downregulated TNF-α through Ca2+, Akt, MAPK, FAK signaling | (16) |
| Human | Spinner vessels | Upregulated IL-6 | (17) | |
| Human | Spinner flask | Suppressed T cell proliferation | (18) | |
| Rat | ALN-reactor | Inhibited inflammatory markers (TNF-α, IFN-γ) and induced other cytokines (IL-1, IL-6, IL-12) | (19) | |
| Tensile stress | Human | PDMS-based custom-made device | Upregulated inflammatory markers (IL-6, TNF-α) | (23) |
| Human | Flexercell FX-4000TM strain unit | Upregulated pro-inflammatory markers (IL-6, IL-8) | (24) | |
| Human | Custom-made pressure chamber | Upregulated Piezo1 and osteogenic maker genes expression through ERK, p38 signaling | (30) | |
| Mouse | Flexcell Fx-4000T tension unit | TNF-α endocytosis-derived MSC homeostasis | (32) | |
| HP | Human | Custom pressure bioreactor | Upregulated COX-2, depending on HP magnitude and frequency | (31) |
MSC: mesenchymal stromal cell, COX-2: cyclooxygenase-2, HO-1: heme oxygenase-1, PGE2: prostaglandin E2, TNF-α: tumor necrosis factor-alpha, MAPK: mitogen-activated protein kinase, FAK: focal adhesion kinase, ALN: artificial lymph node, IFN-γ: interferon-gamma, IL-1: interleukin-1, PDMS: polydimethylsiloxane, IL-6: interleukin-6, IL-8: interleukin-8, IL-12: interleukin-12, Piezo1: Piezo-type mechanosensitive ion channel component 1, ERK: extracellular-regulated kinase, HP: hydrostatic pressure.
Table 2
| Type of ECM | Method | MSC source | Immunomodulation | References |
|---|---|---|---|---|
| Spheroid | Hanging drop | Human bone marrow | Increased secretion of TSG-6, STC-1, LIF | (35) |
| Hanging drop | Human bone marrow | High anti-inflammatory effect through TSG-6, TRAIL, IL-24 expression and PGE2 secretion | (37) | |
| Hanging drop | Human umbilical cord | Increased expression of IL-6, MCP-1, LIF, G-CSF, SDF-1α, and decreased levels of TGF-β1, TGF-β2 in 3D culture conditions compared to 2D culture | (36) | |
| Hydrogel | Human bone marrow | Increased expression of IDO1, GAL9, PTGS2, IL1RN, HGF, IL-10 in hydrogel spheroids compared to 2D | (38) | |
| Floating | Human bone marrow | Increased HO-1, PTGES, COX-2 gene expression and PGE2 secretion | (40) | |
| Spinner flask culture | Human umbilical cord | 3D MSC-derived exosomes reduced the systemic levels of pro-inflammatory cytokines (IL-6, TNF-α) in vivo at late stage of wound healing; increased secretion of IL-10, TGF-β, TNF-α or upregulated IL-1α and VEGF-α in vitro compared to 2D MSC-derived exosome | (42, 43) | |
| Scaffold | Decellularized liver scaffold | Human umbilical cord | Increased PGE2 secretion by the 3D group and decreased IFN-γ | (44) |
| Silk fibroin nanofiber | Human bone marrow | MSC on silk fibroin scaffolds elevated IDO1, COX-2, PGE2 expression and reduced mortality in sepsis-induced animal models | (47) | |
| PCL/SF fibrous scaffold | Human bone marrow | M2 macrophage polarization and inhibited expression of cytokines (IL-1β, CXCL11, IL-10, IL1R2, TGF-β1) | (45) | |
| PLLA fibrous scaffold (PLLA fibrous scaffold, aligned fiber) | Human adipose tissue | Aligned fiber MSC enhanced the expression and secretion levels of TSG-6, COX-2 compared to random fiber MSCs, probably due to increased activation of YAP/TAZ signaling | (48) | |
| PCL fibrous scaffold (PCL fibrous random, mesh, aligned like fiber scaffold) | Rat adipose tissue | Increased gene expression and cytokine secretion of COX-2, TSG-6, iNOS, PGE2, TGF-β in MSCs from random, aligned, and mesh-patterned scaffolds compared to microplates | (46) | |
| Porous scaffold (collagen, chitosan, PLGA substrate) | Human umbilical cord | Increased levels of IL1A, IL1B, IL1RN, IL6ST, VEGF, HGF genes and PTGS2, IL1RN, IL-1β proteins | (50) | |
| Porous scaffold (polystyrene) | Human bone marrow | MSCs in scaffold decreased IL-6, MCP-1 secretion and increased PGE2, TSG-6 levels | (49) | |
| Rigidity | Stiffness | Mouse MSC | Increased MSC protein production of inflammatory modulators (IDO1 and PGE2) when hydrogel stiffness increased from 3 to 18 kPa | (51) |
| Stiffness | Human bone marrow | TNF-α-primed MSCs in soft ECM (∼2 kPa) mimicking bone marrow maximized the ability of MSCs to produce paracrine factors that induced chemotaxis upon inflammatory stimulation | (52) | |
| Elasticity | Human bone marrow | More VEGF secreted by MSC in stiffer gels, while PGE2 secretion was highest in more compliant gels | (53) | |
| Functionalization of substrate surface | Thermoplastic polyurethane plates with grid-like cavities or no structure | Human bone marrow | Enhanced secretion levels of immune modulators (PGE2, IL1RA) by MSCs on grid-like structure | (44) |
| RGD peptide | Human bone marrow | T/DOP-IL-4/CG-RGD surface induced the conversion of macrophages to the M2 and increased the expression of IL-10 compared to T/DOP/CG | (45) |
MSC: mesenchymal stromal cell, ECM: extracellular matrix, TSG-6: tumor necrosis factor-alpha (TNF-α)-stimulated gene protein-6, STC-1: stanniocalcin-1, LIF: leukemia inhibitory factor, TRAIL: tumor necrosis factor-related apoptosis-inducing ligand, IL-24: interleukin-24, PGE2: prostaglandin E2, IL-6: interleukin-6, MCP-1: monocyte chemoattractant protein-1, G-CSF: granulocyte-colony stimulating factor, SDF-1α: stromal cell-derived factor 1 alpha, TGF-β1: transforming growth factor-beta 1, TGF-β2: transforming growth factor-beta 2, 3D: three-dimensional, 2D: two-dimensional, IDO1: indoleamine 2,3-dioxygenase, GAL9: galectin-9, PTGS2: prostaglandin-endoperoxide synthase 2/cyclooxygenase 2, IL1RN: interleukin-1 receptor antagonist, HGF: hepatocyte growth factor, IL-10: interleukin-10, HO-1: heme oxygenase-1, PTGES: prostaglandin E synthase, COX-2: cyclooxygenase-2, TNF-α: tumor necrosis factor-alpha, IL-10: interleukin-10, IL-1α: interleukin-1 alpha, VEGF-α: vascular endothelial growth factor-alpha, TGF-β: transforming growth factor-beta, IFN-γ: interferon-gamma, PCL: poly (ε-caprolactone), SF: silk fibroin, IL-1β: interleukin-1 beta, CXCL11: C-X-C motif chemokine ligand 11, IL1R2: interleukin-1 receptor type 2, PLLA: poly (L-lactic acid), YAP: Yes-associated protein, TAZ: transcriptional coactivator with a PDZ-binding domain, iNOS: inducible nitric oxide synthase, PLGA: poly (lactic-co-glycolic acid), IL6ST: interleukin-6 cytokine family signal transducer, IL1RA: interleukin-1 receptor antagonist (encoded by the IL1RN gene), RGD: arginine-glycine-aspartate, T: titanium, DOP: poly(dopamine), IL-4: interleukin-4, CG: carboxymethyl chitosan hydrogel layer, IL1A: interleukin 1 alpha, IL1B: interleukin 1 beta, VEGF: vascular endothelial growth factor.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download