Abstract
Adrenal incidentalomas represent an increasingly common clinical conundrum with significant implications for patients. The revised 2023 European Society of Endocrinology (ESE) guideline incorporates cutting-edge evidence for managing adrenal incidentalomas. This paper provides a concise review of the updated contents of the revised guideline. In the 2023 guideline, in patients without signs and symptoms of overt Cushing’s syndrome, a post-dexamethasone cortisol level above 50 nmol/L (>1.8 μg/dL) should be considered as mild autonomous cortisol secretion. Regarding the criteria of benign adrenal adenomas, a homogeneous adrenal mass with ≤10 Hounsfield units on non-contrast computed tomography requires no further follow-up, irrespective of its size. The updated guideline also discusses steroid metabolomics using tandem mass spectrometry to discriminate malignancy. It underscores the importance of high-volume surgeons performing adrenalectomy and emphasizes the pivotal role of a multidisciplinary team approach in deciding the treatment plan for indeterminate adrenal masses. The guideline advocates for more proactive surgical treatment for indeterminate adrenal masses in young patients (<40 years) and pregnant women. This review of the 2023 ESE guideline underscores the ongoing evolution of the adrenal incidentaloma management landscape, emphasizing the need for further research and adaptation of diagnostic and therapeutic strategies.
Adrenal incidentalomas, which are tumors of the adrenal gland discovered incidentally during radiologic examinations for other purposes, have emerged as an area of interest in the medical field. The incidence of adrenal incidentalomas has increased dramatically in recent years due to the widespread use of crosssectional imaging scans [1]. Although the majority of these tumors are benign and nonfunctioning, certain types, including adrenal cortical carcinoma, pheochromocytoma, and functioning tumors, can significantly affect patients’ health and necessitate therapeutic interventions.
Given the heterogeneous clinical landscape of adrenal incidentalomas, it is crucial to develop comprehensive, evidencebased guidelines to guide the appropriate diagnostic procedures, therapeutic management, and long-term monitoring of patients with adrenal incidentalomas. Since the National Institutes of Health issued the first guideline for managing adrenal incidentalomas, several other guidelines have been published by various organizations: the American Association of Clinical Endocrinologists (AACE) and American Association of Endocrine Surgeons (2009) [2], Canadian Urological Association (2011) [3], Italian Association of Clinical Endocrinologists (2011) [4], European Society of Endocrinology (ESE) (2016) [5], and Korean Endocrine Society (KES) (2017) [6]. Most of these guidelines concur on the initial hormonal and radiological assessment of adrenal incidentalomas. However, the management strategies for adrenal tumors with autonomous cortisol secretion and the follow-up protocols for non-operated tumors have evolved in light of emerging evidence.
The recently published 2023 ESE guideline has updated the previous 2016 version, specifically in terms of the assessment of malignancy risk, the definition and management of mild autonomous cortisol secretion (MACS), and the follow-up strategies for adrenal tumors [5,7]. Herein, we aim to concisely review current guidelines on adrenal incidentalomas, focusing on the recently published 2023 ESE guideline.
A comprehensive flow diagram detailing the management of patients with adrenal incidentaloma is presented in Fig. 1. The key updates in the 2023 ESE guideline, as well as their differences with the 2016 ESE guideline and the 2017 KES guideline, are delineated in Table 1.
All guidelines recommend a clinical assessment for symptoms and signs of adrenal hormone excess, as well as a 1-mg overnight dexamethasone suppression test (DST). A notable addition to the 2023 guideline is the statement that in frail patients with limited life expectancy, the DST may not be necessary. This is based on some evidence of increasing serum cortisol levels after a DST with age [8] and diminishing the clinical significance of MACS in patients over 65 years of age [9].
The 2016 ESE and 2017 KES guidelines recommend measuring plasma free or urinary fractionated metanephrines in all patients with adrenal incidentalomas to exclude pheochromocytoma. However, recent studies have demonstrated that an adrenal mass with ≤10 Hounsfield units (HU) on unenhanced computed tomography (CT) is unlikely to be pheochromocytoma [10,11]. Thus, the 2023 ESE guideline does not recommend measuring plasma free or urinary fractionated metanephrines in patients with adrenal incidentaloma with ≤10 HU on unenhanced imaging. All guidelines recommend obtaining the plasma aldosterone/renin ratio in patients with hypertension or unexplained hypokalemia.
In the 2016 ESE and the 2017 KES guidelines, the 1-mg overnight DST results are categorized as follows: cortisol levels of 1.8 μg/dL or lower are considered normal, levels between 1.9 and 5.0 μg/dL are classified as possible autonomous cortisol secretion, and levels exceeding 5.0 μg/dL are labeled as autonomous cortisol secretion [5,6]. However, evidence indicates a pattern of relationship between increased serum cortisol levels after DST and the risk of comorbidities and mortality was not linear [8,9,12]. Therefore, the 2023 ESE guideline defines MACS as cases where serum cortisol levels after the 1-mg DST exceed 1.8 μg/dL without any further stratification by degree of post-dexamethasone cortisol level. Moreover, the 2023 ESE guideline recommends testing adrenocorticotropic hormone (ACTH)-independency by demonstrating suppressed or low-normal morning plasma ACTH levels and a repeat DST to confirm MACS. Although the updated guideline emphasizes the confirmation of ACTH-independency, the diagnosis of MACS still relies on the 1-mg DST. Therefore, it is necessary to develop new diagnostic markers for MACS.
In patients with MACS, a careful second clinical evaluation might be necessary to search for signs of overt Cushing’s that may have been overlooked at first examination. Recent studies have shown that MACS is associated with an increased prevalence of cardiometabolic diseases such as diabetes mellitus and dyslipidemia, ranging from 15% to 40% [9,12-14]. Hence, all guidelines recommend clinical decision-making based on age, general conditions, the patient’s preference, and the presence of comorbidities potentially attributable to hypercortisolism. However, debate continues regarding whether surgical treatment is necessary for MACS. As stated in the 2023 ESE guideline, there is currently a lack of randomized controlled trials for cardiovascular outcomes or mortality comparing conservative and surgical treatment, although some studies have demonstrated a decrease in comorbidities following surgical treatment [7,15,16]. The 2023 ESE guideline recommends a multidisciplinary team approach to determine the necessity of surgical intervention in MACS patients. Thus, there is a pressing need for randomized controlled trials to develop treatment strategies for MACS and to discern which MACS cases necessitate surgical intervention.
The KES guideline recommends annual hormone tests in patients with initially nonfunctioning tumors for a minimum of 4 to 5 years, consistent with the 2009 AACE guideline [2,6]. This is based on the fact that approximately 28% of nonfunctioning tumors can develop MACS during a follow-up period of 2.9 years [17]. However, both ESE guidelines do not recommend additional functional tests for confirmed nonfunctioning adrenal tumors during the initial hormone work-up unless new clinical symptoms are identified. The 2023 ESE guideline states that MACS rarely develops into overt Cushing’s syndrome (<1%) and that false-positive DST results are possible. Nevertheless, long-term studies with annual biochemical tests in patients with adrenal incidentalomas are needed to justify the single initial hormone evaluation.
The 2016 ESE guideline recommends against additional imaging follow-up for adrenal incidentalomas measuring <4 cm with ≤10 HU on unenhanced imaging [5]. The 2017 KES guideline recommends a comprehensive evaluation for malignancy risk, including size, HU on unenhanced imaging, washout characteristics, and homogeneity on CT [6]. However, it suggests no definite cutoff values for excluding malignancy.
In the 2023 ESE guideline, the size criterion (<4 cm) has been removed, and it is now advised not to perform additional imaging follow-up for homogeneous adrenal masses with ≤10 HU, irrespective of size [7]. Evidence indicates that values of ≤10 HU are only found in benign adrenal tumors. This change is intended to reduce the socio-economic costs associated with excessive follow-up examinations for benign adrenal masses [18,19].
The KES guideline recommends follow-up examinations at intervals of 3 to 6 months for evaluation of malignancy, followed by annual examinations for the next 1 to 2 years, and does not specify the follow-up interval or duration based on the risk of malignancy [6].
One of the changes in the 2023 ESE guideline is the recommendation of immediate additional imaging for nonfunctioning adrenal tumors that are homogeneous, less than 4 cm in size, and have 11 to 20 HU [7]. If additional imaging confirms the adrenal tumor as benign, no further follow-up is recommended. Performing a follow-up non-contrast CT scan 12 months later could be an alternative approach. This is supported by data indicating that over 90% of homogeneous adrenal tumors, in the absence of extra-adrenal malignancy, are benign if the HU value is below 20 [17,20,21].
However, the optimal second-line imaging methods for determining malignancy have not been clarified. Thus, further studies are needed to identify additional imaging methods to be incorporated into the decision-making process.
The KES guideline recommends follow-up at 3 to 6 months after the initial imaging, continuing for 1 to 2 years regardless of imaging features. In the 2016 ESE guideline, three options were suggested for indeterminate lesions: immediate additional imaging, interval imaging at 6 to 12 months, and surgery without delay [5]. However, the 2023 guideline suggests an individualized approach involving a multidisciplinary team but explicitly states that an immediate imaging work-up is preferred over an interval imaging follow-up for indeterminate adrenal tumors that have not undergone surgical treatment [7]. This reflects an effort to perform more accurate initial assessments, thereby saving costs and time associated with unnecessary follow-up examinations.
If a patient chooses not to undergo adrenalectomy, one repeat non-contrast CT or magnetic resonance imaging after 6 to 12 months is suggested. Surgical treatment is considered if the mass grows significantly during this period. The KES guideline defines a significant increase in size as an increase of >1 cm in the maximum tumor diameter [6]. However, in the ESE guideline, growth suggestive of malignancy is indicated by an increase of >20% and ≥5 mm in the maximum tumor diameter [7]. If lesions grow below this threshold, additional imaging might be considered after 6 to 12 months.
However, there are still no recommendations on the follow-up duration. Hence, we need updates that consider individual risk groups and take into account factors such as HU values, size, and homogeneity of adrenal tumors.
The 2017 KES guideline recommends measuring sex hormones and steroid precursors in all patients presenting with imaging findings suspicious of malignancy to ascertain potential hormonal overproduction and utilizing these parameters as tumor markers [6]. However, the 2017 KES guideline does not address the utility of urine or plasma steroid profiles using tandem mass spectrometry.
With advances in mass spectrometry, numerous attempts have been made to analyze steroid profiles in order to discriminate malignant lesions or subtypes of adrenal tumors [20,22-27]. Urinary steroid profiles have demonstrated the potential to distinguish between benign and malignant adrenal tumors [23,27]. The integration of urinary steroid profiles with CT image findings led to even higher sensitivity and specificity for malignancy in a prospective study [20]. In addition to the 2016 ESE guideline, which highlighted the potential benefits of measuring sex steroids and steroid precursors, the updated guideline now further suggests that, ideally, steroid metabolomics could be conducted using tandem mass spectrometry for a more comprehensive and accurate diagnosis. However, steroid profiling is not widely available in routine practice.
All guidelines universally recommend that an experienced adrenal surgeon should perform adrenalectomy [5-7]. However, the 2023 ESE guideline further elaborates on this point by introducing the term “high-volume surgeon” and providing specific criteria for what constitutes a high-volume surgeon. The ESE panel defines a high-volume surgeon as one performing a minimum of 12 adrenalectomies per year and considers a surgeon managing over 20 cases as desirable [7].
Another distinctive feature of the 2023 ESE guideline is the emphasized recommendation for a minimally invasive approach to adrenalectomy. The 2023 ESE guideline recommends adopting a minimally invasive surgical approach for benign adrenal tumors requiring surgery due to hormone excess [7]. If the radiological findings are suspicious of malignancy and the size of the mass is ≤6 cm without local invasion, minimally invasive adrenalectomy is recommended [7]. Another notable update is the emphasis on a multidisciplinary team approach when considering surgical treatments.
The 2023 ESE guideline recommends that patients with MACS should receive perioperative stress-dose glucocorticoid treatment and should be followed up by an endocrinologist until the hypothalamic-pituitary-adrenal axis recovers.
All guidelines recommend that bilateral adrenal incidentalomas should undergo the same clinical, radiological, and hormonal evaluations as unilateral adrenal incidentalomas. This new approach to bilateral diseases is suggested in the 2023 ESE guideline in cases with the following radiological and hormonal results: (1) bilateral (macronodular) hyperplasia; (2) bilateral adrenal adenomas; (3) two morphologically similar but non-adenoma-like adrenal masses; (4) two morphologically different adrenal masses. In particular, the assessment of comorbidities attributable to cortisol excess is recommended in patients with bilateral macronodular hyperplasia.
Similar to previous guidelines, the 2023 ESE guideline recommends the urgent assessment of adrenal incidentalomas in pregnant women and patients <40 years of age due to a higher risk of malignancy and hormone excess. However, a new recommendation has been made for surgical removal in cases of an indeterminate mass in children, adolescents, pregnant women, and adults under 40 years of age.
The management of adrenal incidentalomas has significantly advanced over the years, with updated guidelines aiming to optimize diagnostic processes and therapeutic interventions. The 2023 ESE guideline, in particular, highlights the need for individualized approaches over standardized ones, introducing key modifications in hormonal evaluations, the assessment of malignancy risk, and surgical treatment. The definition and management of MACS have been refined, with the focus shifted toward an integrated understanding of ACTH-independency, patients’ age, comorbidities attributable to cortisol excess, and preferences. This personalized approach is also seen in the appraisal of malignancy risk, focusing on HU values and tumor homogeneity, irrespective of size. The role of steroid metabolomics in diagnosis is increasingly acknowledged, although its use is yet to be mainstreamed. Importantly, the guideline recommends that surgical interventions should be performed by experienced surgeons using minimally invasive techniques when suitable. The guidelines also stress the importance of a multidisciplinary team approach in managing these cases.
Overall, these updated guidelines pave the way towards more accurate diagnosis and effective management of adrenal incidentalomas, potentially reducing unnecessary examinations and thereby easing the socio-economic burden incurred by these masses. Further research, including randomized controlled trials, is needed to strengthen these recommendations and to establish more refined strategies for treating adrenal incidentalomas.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare of the Republic of Korea (Project No. HI21C0032 and HI22C0049).
REFERENCES
1. Ebbehoj A, Li D, Kaur RJ, Zhang C, Singh S, Li T, et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol. 2020; 8:894–902.
2. Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, et al. The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009; 15 Suppl 1:1–20.
3. Kapoor A, Morris T, Rebello R. Guidelines for the management of the incidentally discovered adrenal mass. Can Urol Assoc J. 2011; 5:241–7.
4. Terzolo M, Stigliano A, Chiodini I, Loli P, Furlani L, Arnaldi G, et al. AME position statement on adrenal incidentaloma. Eur J Endocrinol. 2011; 164:851–70.
5. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology clinical practice guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016; 175:G1–34.
6. Lee JM, Kim MK, Ko SH, Koh JM, Kim BY, Kim SW, et al. Clinical guidelines for the management of adrenal incidentaloma. Endocrinol Metab (Seoul). 2017; 32:200–18.
7. Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2023; 189:G1–42.
8. Prete A, Subramanian A, Bancos I, Chortis V, Tsagarakis S, Lang K, et al. Cardiometabolic disease burden and steroid excretion in benign adrenal tumors: a cross-sectional multicenter study. Ann Intern Med. 2022; 175:325–34.
9. Deutschbein T, Reimondo G, Di Dalmazi G, Bancos I, Patrova J, Vassiliadi DA, et al. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: an international, retrospective, cohort study. Lancet Diabetes Endocrinol. 2022; 10:499–508.
10. Buitenwerf E, Korteweg T, Visser A, Haag CM, Feelders RA, Timmers HJ, et al. Unenhanced CT imaging is highly sensitive to exclude pheochromocytoma: a multicenter study. Eur J Endocrinol. 2018; 178:431–7.
11. Canu L, Van Hemert JA, Kerstens MN, Hartman RP, Khanna A, Kraljevic I, et al. CT characteristics of pheochromocytoma: relevance for the evaluation of adrenal incidentaloma. J Clin Endocrinol Metab. 2019; 104:312–8.
12. Kjellbom A, Lindgren O, Puvaneswaralingam S, Londahl M, Olsen H. Association between mortality and levels of autonomous cortisol secretion by adrenal incidentalomas: a cohort study. Ann Intern Med. 2021; 174:1041–9.
13. Di Dalmazi G, Vicennati V, Rinaldi E, Morselli-Labate AM, Giampalma E, Mosconi C, et al. Progressively increased patterns of subclinical cortisol hypersecretion in adrenal incidentalomas differently predict major metabolic and cardiovascular outcomes: a large cross-sectional study. Eur J Endocrinol. 2012; 166:669–77.
14. Sbardella E, Minnetti M, D’Aluisio D, Rizza L, Di Giorgio MR, Vinci F, et al. Cardiovascular features of possible autonomous cortisol secretion in patients with adrenal incidentalomas. Eur J Endocrinol. 2018; 178:501–11.
15. Chiodini I, Morelli V, Salcuni AS, Eller-Vainicher C, Torlontano M, Coletti F, et al. Beneficial metabolic effects of prompt surgical treatment in patients with an adrenal incidentaloma causing biochemical hypercortisolism. J Clin Endocrinol Metab. 2010; 95:2736–45.
16. Iacobone M, Citton M, Viel G, Boetto R, Bonadio I, Mondi I, et al. Adrenalectomy may improve cardiovascular and metabolic impairment and ameliorate quality of life in patients with adrenal incidentalomas and subclinical Cushing’s syndrome. Surgery. 2012; 152:991–7.
17. Hong AR, Kim JH, Park KS, Kim KY, Lee JH, Kong SH, et al. Optimal follow-up strategies for adrenal incidentalomas: reappraisal of the 2016 ESE-ENSAT guidelines in real clinical practice. Eur J Endocrinol. 2017; 177:475–83.
18. Dinnes J, Bancos I, Ferrante di Ruffano L, Chortis V, Davenport C, Bayliss S, et al. Management of endocrine disease: imaging for the diagnosis of malignancy in incidentally discovered adrenal masses: a systematic review and meta-analysis. Eur J Endocrinol. 2016; 175:R51–64.
19. Iniguez-Ariza NM, Kohlenberg JD, Delivanis DA, Hartman RP, Dean DS, Thomas MA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017; 2:30–9.
20. Bancos I, Taylor AE, Chortis V, Sitch AJ, Jenkinson C, Davidge-Pitts CJ, et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINEACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 2020; 8:773–81.
21. Marty M, Gaye D, Perez P, Auder C, Nunes ML, Ferriere A, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018; 178:439–46.
22. Rossi C, Cicalini I, Verrocchio S, Di Dalmazi G, Federici L, Bucci I. The potential of steroid profiling by mass spectrometry in the management of adrenocortical carcinoma. Biomedicines. 2020; 8:314.
23. Bancos I, Arlt W. Diagnosis of a malignant adrenal mass: the role of urinary steroid metabolite profiling. Curr Opin Endocrinol Diabetes Obes. 2017; 24:200–7.
24. Kerkhofs TM, Kerstens MN, Kema IP, Willems TP, Haak HR. Diagnostic value of urinary steroid profiling in the evaluation of adrenal tumors. Horm Cancer. 2015; 6:168–75.
25. Schweitzer S, Kunz M, Kurlbaum M, Vey J, Kendl S, Deutschbein T, et al. Plasma steroid metabolome profiling for the diagnosis of adrenocortical carcinoma. Eur J Endocrinol. 2019; 180:117–25.
26. Ku EJ, Lee C, Shim J, Lee S, Kim KA, Kim SW, et al. Metabolic subtyping of adrenal tumors: prospective multi-center cohort study in Korea. Endocrinol Metab (Seoul). 2021; 36:1131–41.
27. Arlt W, Biehl M, Taylor AE, Hahner S, Libe R, Hughes BA, et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab. 2011; 96:3775–84.
Fig. 1.
Flow diagram on the management of patients with adrenal incidentaloma. Modified from Fassnacht et al. [7]. MDT, multidisciplinary team; ACTH, adrenocorticotropic hormone. aOnly in adrenal tumors with >10 Hounsfield units (HU) on unenhanced computed tomography (CT); bOnly in patients with hypertension or hypokalemia; cOnly in patients with findings suggestive of adrenocortical carcinoma; dIndeterminate adrenal mass: homogeneous with 11 to 20 HU and tumor ≥4 cm, homogeneous with >20 HU and tumor <4 cm, and heterogeneous tumors <4 cm; eFluorodeoxyglucose positron emission tomography/CT, adrenal magnetic resonance imaging (MRI) with chemical shift, or washout CT.
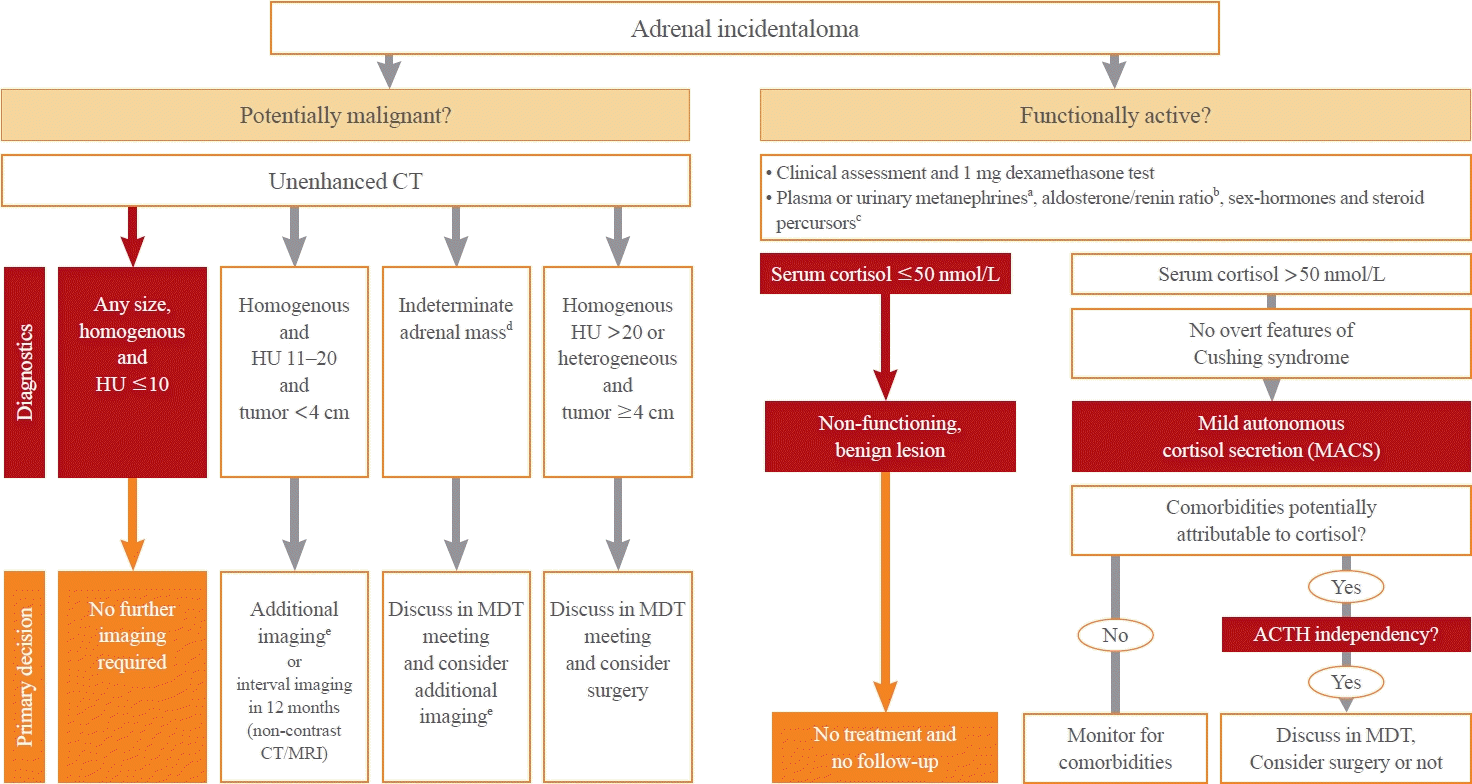
Table 1.
Key Updates to the 2023 ESE Guideline
| Remarks | 2023 ESE guideline | 2016 ESE guideline | 2017 KES guideline | |
|---|---|---|---|---|
| Category for serum cortisol after 1-mg DST | Recommend: MACSa: patients without features of overt Cushing’s syndrome with serum cortisol after DST >50 nmol/L (>1.8 μg/dL)a | Suggest: possible ACSa: serum cortisol after DST between 51 and 138 nmol/L (1.9–5.0 μg/dL) | Similar to the 2016 ESE guidelines | |
| Confirm ACTH-independency, repeat DST, consider conditions that alter the results | ACSa: serum cortisol after DST >138 nmol/L (>5.0 μg/dL) | |||
| Additional biochemical tests to assess the degree of cortisol secretion might be usefula. | Additional biochemical tests might be requireda. | |||
| Treatment for patients with MACS | Recommenda: discussing surgery with the patient. Consider age, sex, general health, degree and persistence of non-suppressible cortisol after dexamethasone, severity of comorbidities, and patient’s preference. The proposal to perform surgery should be established within an expert MDTa | Suggesta: an individualized approach for adrenal surgery. Consider age, degree of cortisol excess, general health, comorbidities, and patient’s preference. In all patients considered for surgery, ACTH-independency of cortisol excess should be confirmed. | NA | |
| Measurement of sex hormone and steroid precursors | Suggest: ideally, use multi-steroid profiling by tandem mass spectrometrya in patients in whom an adrenocortical carcinoma is suspected. | Suggesta: in patients with clinical or imaging features suggestive of adrenocortical carcinoma | Recommenda: all patients suspected of having adrenal cancer. | |
| Benign criteria for no further imaging | Recommenda: homogeneous appearance and ≤10 HU on non-contrast CT | Suggesta: homogeneous appearance, smaller than 4 cm and ≤10 HU on non-contrast CT | Homogeneous appearance, smaller than 4 cma and ≤10 HU on non-contrast CT+contrast washout in delayed images of contrast CT | |
| Management of indeterminate adrenal nodules | (1) Adrenal mass with unenhanced HU between 11 and 20 and <4 cma | Three optionsa: | Recommend: follow-up imaging in 3–6 months after the initial study and continuing for 1–2 yearsa | |
| Suggest: immediate additional imaging to avoid any follow-up imaging. | (1) Immediate additional imaging with another modality | |||
| Optional: interval imaging in 12 months by non-contrast CT (or MRI) | (2) Interval imaging in 6 to 12 months (non-contrast CT or MRI) | |||
| (2) Adrenal mass ≥4 cm and unenhanced >20 HUa | (3) Surgery without further delay. | Consider adrenalectomya if the mass enlarges by 1 cma or more and/or changes its appearance during observation | ||
| Suggest: MDT, immediate surgery/staging | ||||
| Optional: follow-up imaging in 6–12 months | ||||
| (3) Adrenal mass ≥4 cm with unenhanced HU 11–20; or <4 cm with unenhanced HU >20; or tumor size <4 cm with heterogeneous appearance | ||||
| Suggest: individualized approach in MDT | ||||
| Surgical treatment | Recommend: surgery by an expert high-volume adrenal surgeona in patients suspicious of malignancy | NA | NA | |
| Suggest: surgical resection if indeterminate adrenal mass on imaging in children, adolescents, pregnant women and adults <40 years of agea. | ||||
| Hormone follow-up of nonfunctioning tumors at initial evaluation | Recommend: againsta repeated hormonal work-up unless new clinical signs of endocrine activity appear or comorbidities worsen | Suggest: againsta repeated hormonal work-up unless new clinical signs of endocrine activity appear or comorbidities worsen | Recommenda: annual hormone tests for 4–5 years | |
| Follow-up of patients with MACS | Recommend: only annual re-assessment of comorbidities potentially attributable to cortisol. | Suggest: annual clinical re-assessment for comorbidities potentially related to cortisol excess. Based on the outcome of this evaluation the potential benefit of surgery should be considered. | Recommend: annual hormone tests for 4–5 years | |
| If these comorbidities develop or worsen, referral to an endocrinologist. | ||||
| Approach to bilateral adrenal incidentaloma | Suggest following four-option schemaa (1) bilateral (macronodular) hyperplasia, (2) bilateral adrenal adenomas, (3) two morphologically similar, but non-adenoma-like adrenal masses, (4) two morphologically different adrenal masses. | The same applies to the assessment of comorbidities that might be related to ACS. | Similar to the 2016 ESE guideline | |
| Bilateral (macronodular) hyperplasia or bilateral adenomas: recommend assessment of comorbidities attributable to MACSa | Bilateral hyperplasia without ACS: 17-hydroxyprogesterone | |||
| Otherwise, similar to the 2016 ESE guideline | Bilateral metastases, lymphoma, infiltrative inflammatory disease and hemorrhages: recommend assessment for adrenal insufficiency | |||
| Treatment for bilateral adrenal incidentaloma | (1) Bilateral hyperplasia or bilateral adenomas with MACS: suggest individualized treatment optionsa | Suggesta: the same recommendations for patients with unilateral adrenal incidentalomas | NA | |
| (2) Suggest against bilateral adrenalectomya in patients without clinical signs of overt Cushing’s syndrome | Suggest that bilateral adrenalectomy is not performeda without clinical signs of overt Cushing’s syndrome. | |||
Modified from Fassnacht et al. [7].
ESE, European Society of Endocrinology; KES, Korean Endocrine Society; DST, dexamethasone suppression test; MACS, mild autonomous cortisol secretion; ACTH, adrenocorticotropic hormone; ACS, autonomous cortisol secretion; MDT, multidisciplinary team; NA, not available; HU, Hounsfield unit; CT, computed tomography; MRI, magnetic resonance imaging.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download