Abstract
Solitary fibrous tumors (SFTs) are an uncommon group of neoplasms. The visceral pleura is the most common site of origin of these tumors. The colonic mesentery is an unusual site of origin of SFTs. A pre-operative diagnosis of SFT is challenging as there are no pathognomonic clinical or radiological signs. Most patients reported thus far were diagnosed post-operatively with the aid of immunohistochemical markers. Complete surgical excision is the treatment of choice for SFTs. Recurrences are uncommon. However, they can occasionally show aggressive behavior. In this report, we describe two cases of rare colonic mesentery SFTs.
Solitary fibrous tumors (SFTs) are an uncommon group of mesenchymal neoplasms. They commonly arise from the thoracic pleura.1 These spindle cell tumors are characterized by increased fibrosis and vascularity.1 SFTs can originate from anywhere in the body. However, only a few cases arising from the mesentery have been described.1,2 Here we report two cases of SFTs from the colonic mesentery.
As this was a case report, approval from the Institutional Ethics Committee of the Nizam’s Institute of Medical Sciences, Telangana, India, was not required. Informed consent was taken from the patients and further consent to publish the data regarding the case and relevant photographs was obtained.
A 50-year-old male presented with a gradually progressive abdominal lump over two months. He had intermittent episodes of non-bilious vomiting and lost 8 kg of weight. He underwent bilateral craniotomy for meningioma in 2009 and 2014. During the physical examination, an 8×10 cm firm to hard palpable mass was detected in the left hypochondrium. On ultrasonography (USG) of the abdomen, a hypoechoic solid mass was found in the pancreatic body and tail. Contrast-enhanced computed tomography (CECT) revealed a heterogeneously enhancing mass (16.7 cm craniocaudal, 12.3 cm anteroposterior, 13.4 cm transverse) with lobulated margins and central necrotic components occupying the epigastric and left hypochondriac and left lumbar region (Fig. 1A). An upper gastrointestinal endoscopy showed an extrinsic impression over the body of the stomach. The differential diagnosis included solid pseudo epithelial neoplasm, gastrointestinal stromal tumor (GIST), and neuroendocrine tumor (NET). A core biopsy was recommended to confirm the diagnosis. Surprisingly, the core biopsy revealed an SFT (immunohistochemistry [IHC]: cluster of differentiation-34 [CD34] and signal transducer and activator of transcription 6 [STAT-6] Positive). A large tumor in the lesser sac which appeared to arise from the pancreas involving the transverse colon, mesentery of the transverse colon, and the splenic vein was detected intra-operatively. The tumor was free of the splenic artery, portal, and superior mesenteric vein. The patient underwent distal pancreatectomy and splenectomy with segmental colonic resection and colo-colic anastomosis (Fig. 1B). On histopathological examination (HPE) of the specimen, spindle-shaped tumor cells with elongated nuclei, some areas of myxoid degeneration, and minimal mitoses were seen growing from the transverse colon mesentery. Immunohistochemistry confirmed that the tumor cells were positive for CD34 and STAT-6 (Fig. 2). The tumor cells were negative for smooth muscle actin, Ki67, and cluster of differentiation 117 (CD117). The post-operative course was uneventful. The patient did not receive any adjuvant therapy. There was no tumor recurrence observed at the 36-month follow-up.
A 55-year-old male presented with complaints of abdominal fullness associated with constipation and anorexia for three months, in addition to a 9 kg weight loss. A firm palpable non-tender mass occupying the hypogastric and umbilical regions of the abdomen with side-to-side mobility was observed on clinical examination. On rectal examination, there was fullness at the 6 óclock position, and normal mucosa was felt. A USG of the abdomen revealed a large mixed echogenic mass in the hypogastric region. CECT of the abdomen and pelvis revealed a large heterogeneous, enhancing (size: 18 cm craniocaudal, 15 cm anteroposterior, 12 cm transverse), well-circumscribed mass with lost fat planes and left lateral sigmoid colon (Fig. 3A). The bowel loops were displaced peripherally and the urinary bladder anteriorly. Colonoscopy revealed a normal rectosigmoid mucosa.
GIST and soft tissue sarcoma were considered in the differential diagnosis. Intra-operatively, a large tumor was seen in the rectosigmoid mesentery. The patient underwent a rectosigmoid resection and Hartman’s procedure (Fig. 3B). The patient required multiple packed red blood cell transfusions during surgery. The post-operative course was uneventful. HPE revealed an encapsulated tumor comprising hypercellular and hypocellular areas with hemangiopericytomatous patterns (Fig. 4A, B). On IHC, the tumor was positive for CD34 and STAT6 with 2% Ki67 (Fig. 4C–E). The patient did not receive adjuvant therapy. At the 24 months follow-up, no local or systemic recurrence was noted.
The term SFT replaced the nomenclature of neoplasms that were referred to as hemangiopericytoma 20 years ago.3 SFTs are commonly found in the thoracic cavity, especially in the pleura. However, they can arise from any site in the human body.2 SFTs originating from the mesentery have rarely been reported. A literature search revealed only 15 reported cases involving large bowel mesenteric SFTs (Table 1).2-17 Of these reports, the median age of presentation was 60 years (range: 27–83 years) with a preponderance among males (10/15). These tumors more commonly originated from the left side (rectum and sigmoid colon: 9/15; descending colon 1/15) than the right side of the colon (2/15), with the median size of the lesion being 12 cm (range 6–25 cm). Surgery was the treatment of choice for all patients. One patient was administered pre-operative radiotherapy.11 No patient received any form of adjuvant therapy. CD34 and STAT6 positivity were reported in 12/15 and 3/15 cases, respectively. In 11/15 cases, there was no local or distant recurrence on a median follow-up of 12 months (range 2–252 months).
Pre-operative diagnosis of SFTs is challenging, as there are no pathognomonic clinical or radiological features. Desmoid, inflammatory pseudotumor, mesenteric fibromatosis, leiomyomas, GIST, NETs, fibrosarcoma, and malignant fibrous histiocytoma can mimic SFTs. A definite preoperative diagnosis with core biopsy can rule out SFTs from GIST. Pre-operative imatinib therapy in large C-Kit positive GISTs downsizes the tumor and allows the preservation of adjacent organs. However, there are no definite neoadjuvant therapies to downsize SFTs.9 Nishikawa et al.11 reported a partial response of 32.3% with 50 Gy local irradiation in a giant mesorectal SFT. In the two patients presented in this report, evaluation of pre-operative tumor markers such as the carcinoembryonic antigen or cancer antigen 19-9 (CA 19-9) was not performed, as our clinical diagnosis favored SFTs. IHC studies are vital for confirming the SFT diagnosis. The tumors stain positive for CD34, STAT6, B-cell lymphoma 2 (Bcl2), CD99, beta-catenin, and vimentin markers.14 CD34 is the most commonly accepted marker for SFT. The expression of STAT6 in tumor cells is essential to diagnose SFT if CD34 and CD99 are negative.15 Both our patients were positive for CD34 and STAT6.
SFTs are usually benign and have a good prognosis. However, nuclear atypia, necrosis, increased mitotic activity (>4 mitoses per 10 high power fields), and infiltrative margins are associated with malignant forms. In addition, incomplete resection and more than 10 cm are correlated with local recurrence and metastatic disease. Extra thoracic SFTs should be considered potentially malignant as their behavior is unpredictable.3
Complete surgical excision is the treatment of choice. There is no consensus regarding other treatment modalities. The two patients detailed in the present report were from geographically distant locations and were followed up along with their imaging reports via phone calls and instant messaging platforms. Recurrences can occur as late as 13 years after radical resection, and some tumors can behave aggressively even if there is an absence of primary morphologic evidence of malignancy.3,9 Hence, we planned for close long-term follow- up of our patients.
Colonic mesentery SFTs are unusual in occurrence and usually diagnosed post-operatively. The immunohistochemical markers, CD34 and STAT6 help differentiate SFTs from other mesenchymal tumors. Complete surgical excision is the treatment of choice. Recurrences are uncommon.
REFERENCES
1. Liu JN, Liu Z, Ji PY, Zhang H, Guo SL. 2020; Solitary fibrous tumor of the mesentery: a case report and review of the literature. J Int Med Res. 48:300060520950111. DOI: 10.1177/0300060520950111. PMID: 33050750. PMCID: PMC7710395.

2. Mekel G, Balshan E, Traupman F. 2019; Solitary fibrous tumour of the sigmoid colon mesentery. BMJ Case Rep. 12:e228774. DOI: 10.1136/bcr-2018-228774. PMID: 31068346. PMCID: PMC6506114.

3. Val-Bernal JF, Mayorga M, Fernández F, Parra A, Crespo J, García-Polavieja M. 2014; Solitary fibrous tumor arising from the mesentery of adult patients. Report of two cases and review of the literature. Rom J Morphol Embryol. 55:203–207.
4. Kay S, Warthen HJ. 1953; Hemangiopericytoma of the rectum. Cancer. 6:167–169. DOI: 10.1002/1097-0142(195301)6:1<167::AID-CNCR2820060119>3.0.CO;2-0.

5. Genter B, Mir R, Strauss R, et al. 1982; Hemangiopericytoma of the colon: report of a case and review of literature. Dis Colon Rectum. 25:149–156. DOI: 10.1007/BF02553264. PMID: 7067552.
6. Nakagawa T, Shinoda Y, Masuko Y, et al. 1997; Hemangiopericytoma of the sigmoid mesentery: report of a case with immunohistochemical findings. Surg Today. 27:64–67. DOI: 10.1007/BF01366942. PMID: 9035303.

7. West NJ, Daniels IR, Allum WH. 2004; Haemangiopericytoma of the sigmoid mesentery. Tech Coloproctol. 8:179–181. DOI: 10.1007/s10151-004-0084-2. PMID: 15654526.

8. Balaji R, Ramachandran K, Somanathan T. 2009; A rare case of solitary fibrous tumour of the sigmoid mesocolon: imaging features and review of literature. Cancer Imaging. 9:67–69. DOI: 10.3816/CBC.2009.n.034. PMID: 19661047.
9. Soda H, Kainuma O, Yamamoto H, et al. 2010; Giant intrapelvic solitary fibrous tumor arising from mesorectum. Clin J Gastroenterol. 3:136–139. DOI: 10.1007/s12328-010-0146-0. PMID: 26190119.

10. Medina-Franco H, Cabrera-Mendoza F, Almaguer-Rosales S, Guillén-Pérez F, Chablé-Montero F. 2011; [Mesenteric solitary fibrous tumor: a case report and literature review]. Rev Gastroenterol Mex. 76:186–189. Spanish.
11. Nishikawa T, Takei Y, Teshima S, et al. 2013; A hypervascular malignant solitary fibrous tumor in the mesentery of the rectum with good response to preoperative radiation therapy. Int Cancer Conf J. 2:30–35. DOI: 10.1007/s13691-012-0059-5.

12. Scarlett A, Stillwell R, Deutscher D, Sengupta SK. 2015; 49. Solitary fibrous tumour of transverse mesocolon: An unusual location. Pathology. 47(Suppl 1):S115. DOI: 10.1097/01.PAT.0000461658.78598.52.

13. Ge W, Yu DC, Chen G, Ding YT. 2016; Clinical analysis of 47 cases of solitary fibrous tumor. Oncol Lett. 12:2475–2480. DOI: 10.3892/ol.2016.4967. PMID: 27698815. PMCID: PMC5038456.

14. Keser BN, Kırman ÜN, Aktemur G, Alimoĝlu O. 2019; A rare solitary fibrous tumour of the ascending mesocolon: a case report. Ann R Coll Surg Engl. 101:e108–e110. DOI: 10.1308/rcsann.2019.0031. PMID: 30854871. PMCID: PMC6432965.

15. Chaouch MA, Jerraya H, Dougaz MW, Haloui N, Bouasker I, Nouira R. 2020; A case report of a right mesocolon solitary fibrosis tumour. J Gastrointest Cancer. 51:351–353. DOI: 10.1007/s12029-019-00294-x. PMID: 31407250.

16. Hoseini M, Negahi A, Vosough F, et al. 2020; Solitary fibrous tumor in pelvis extended to transverse mesocolon and peritoneum. Res J Pharm Technol. 13:1943–1950. DOI: 10.5958/0974-360X.2020.00350.9.

17. Sousa M, Proença L, Baldaia H. 2020; Intussusception caused by a colonic solitary fibrous tumor. GE Port J Gastroenterol. 27:59–61. DOI: 10.1159/000499137. PMID: 31970245. PMCID: PMC6959109.

Fig. 1
(A) Contrast-enhanced computed tomography showing a heterogeneously enhancing mass with lobulated margins and central necrotic components. (B) Specimen showing distal pancreatectomy and splenectomy along with the resected segment of the colon.
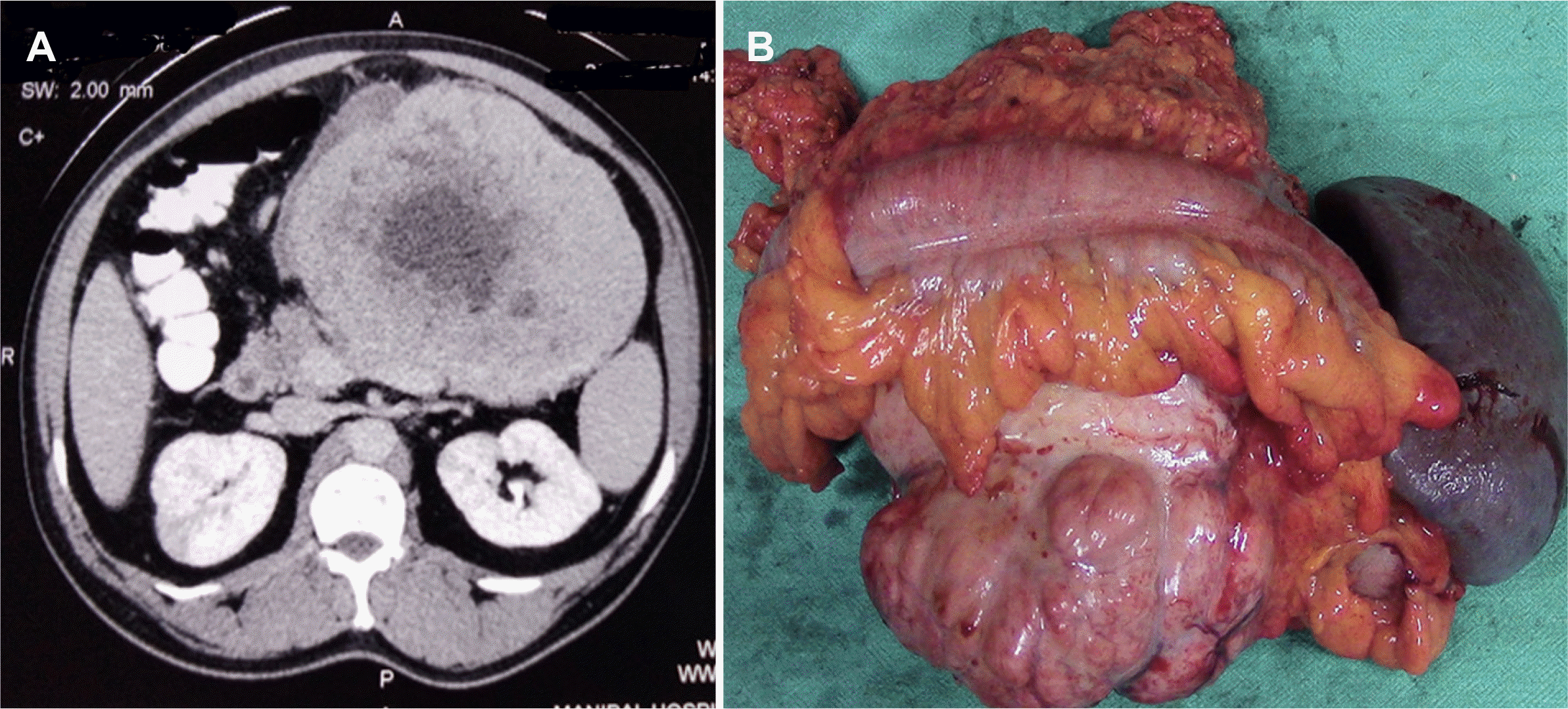
Fig. 2
(A) H&E sections reveal a higher magnification of the cellular area of the tumor showing closely packed cells with little intervening collagenous stroma. Tumor cells have oval to slightly elongated bland nuclei with fine pale chromatin and inconspicuous nucleoli. The cytoplasm is scant to moderate, pale eosinophilic with indistinct borders (×400). (B) Higher magnification of the hypocellular area of the tumor showing prominent hyalinised (keloid-like) collagenous stroma with relatively few tumor cells (×400). Immunohistochemical findings of the tumor. (C) CD34 and (D) STAT6 shows diffuse cytoplasm and nucleus (with some cytoplasmic staining) in the tumor cells respectively (HRP-Polymer; ×400). (E) Immunohistochemical staining with Ki-67 labels occasional tumor cell nuclei indicating low proliferative activity within the tumor (HRP-Polymer; ×400).
Fig. 3
(A) Contrast-enhanced computed tomography showing a heterogeneously enhancing mass occupying the pelvis. (B) Post-operative specimen showing the resected recto-sigmoid colon with the adjacent tumor.
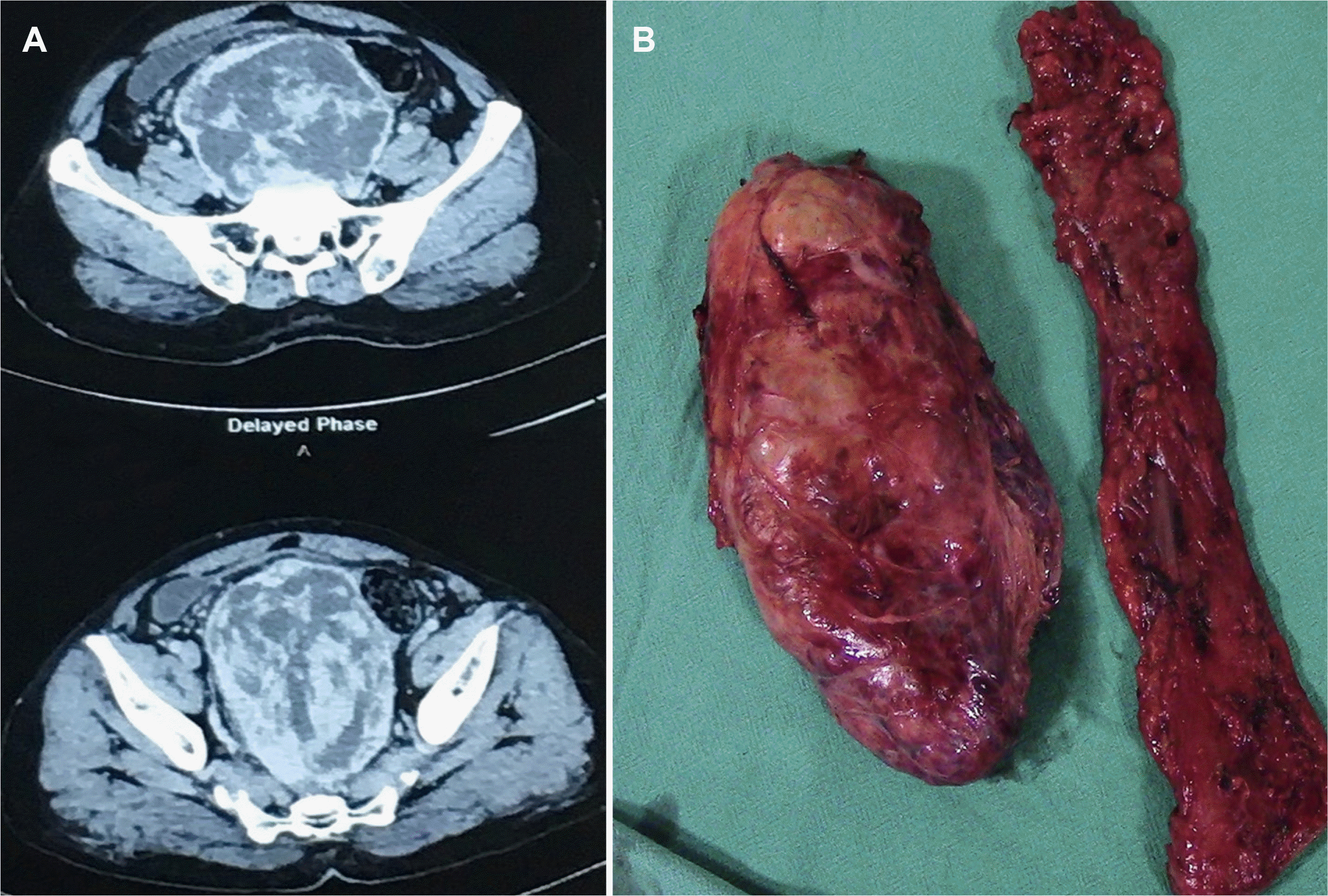
Fig. 4
Hematoxylin and esosin staining. (A) Cells arranged in fascicles separated by collagen (magnification ×100). (B) Cellular lesion with a hemangiopericytomatous pattern; immuno-histochemical staining (magnificantion ×400). (C) Nuclear positivity for STAT6 (HRP-Polymer; ×400). (D) Tumor cell positivity for CD34 (HRP-Polymer; ×400). (E) Low Ki67 index (HRP-Polymer; ×400).

Table 1
List of Cases Reported on Solitary Fibrous Tumor from Mesocolon
| Author, yr | Age in yr/Sex | Max diameter (cm) | Mesenteric (tumor) location | IHC | Ki67 | Mitotic index | F/U | Rec |
|---|---|---|---|---|---|---|---|---|
| Kay et al., 19534 | 75/M | 16 | Rectum | NR | NR | NR | NR | NR |
| Genter et al., 19815 | 31/F | 3 | DC | NR | NR | 1/40 hpf | 12 | No |
| Nakagawa et al., 19976 | 83/M | 10 | SC | Vimentin+ | NR | Absent | 11 | No |
| West et al., 20037 | 61/M | 9.5 | SC | CD34+, focal FactorVIII+ | NR | Absent | NR | NR |
| Balaji et al., 20098 | 68/M | 18 | SC | CD34+ | NR | NR | NR | NR |
| Soda et al., 20109 | 27/F | 16 | Rectum | CD34+, Bcl2+ | NR | Absent | 12 | No |
| Medina-Franco et al., 201110 | 60/F | 16 | TC | CD34+, Bcl2+, vimentin+ | NR | NR | 84 | No |
| Nishikawa et al., 201311 | 36/M | 11.5 | Rectum | CD34+, CD99+, Bcl2+ | 10%a | Lowa | 26 | No |
| Val-Bernal et al., 20143 | 32/M | 13 | SC | CD34+, CD99+, Bcl2+ | NR | 1/50 hpf | 252 | No |
| Scarlett et al., 201512 | 55/F | 17 | TC | CD34+, beta catenin+, CD99+, Bcl2+ | NR | NR | NR | NR |
| Ge et al., 201613 | 50/F | 25 | SC | CD34+ | 5 | No | ||
| Keser et al., 201914 | 35/M | 9.5 | DC | CD34+, STAT6+, Vimentin+, Bcl2+ | Negative | NR | 2 | No |
| Chaouch et al., 201915 | 65/F | 12 | AC | CD34+, CD99+, Bcl2+ | 8% | 5/10 hpf | 24 | No |
| Mekel et al., 20192 | 79/M | 11 | SC | CD34+, Bcl2+ | NR | 5/10 hpf | 18 | No |
| Hoseini et al., 202016 | 60/M | 6 | TC | 12 | No | |||
| Sousa et al., 202017 | 65/M | 8 | Cecum | CD34-, STAT6+, vimentin+ | NR | Low | 12 | No |
| Present study | 50/M | 16.7 | TC | CD34+, STAT6+ | 4% | 2/10 hpf | 36 | No |
| 55/M | 18 | Rectum | CD34+ STAT6+ | 2% | 1/10 hpf | 24 | No |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download