CASE REPORT
A 19-year-old male presented with lower abdominal pain. The symptom had first occurred one and a half years earlier. Ultrasonography (USG) of the abdomen and pelvis performed after the first presentation a year ago revealed a collection in the right pelvic region for which he underwent ultrasound- guided aspiration. After 1 year of asymptomatic period, the patient presented at our center with a recurrence of his symptoms. General, abdominal, and per rectal examinations did not reveal any abnormality. He was evaluated with a contrast- enhanced computed tomography (CECT) scan which revealed multiple, loculated non-enhancing hypodense cystic lesions of different sizes in the pelvis in relation to the rectosigmoid. The largest cyst measured 8.4×4.4 cm in the right hemipelvis, abutting the rectum, redundant sigmoid colon, and the adjacent urinary bladder (
Figs. 1,
2). There was a loss of the fat plane between the cystic lesion and the upper rectal wall.
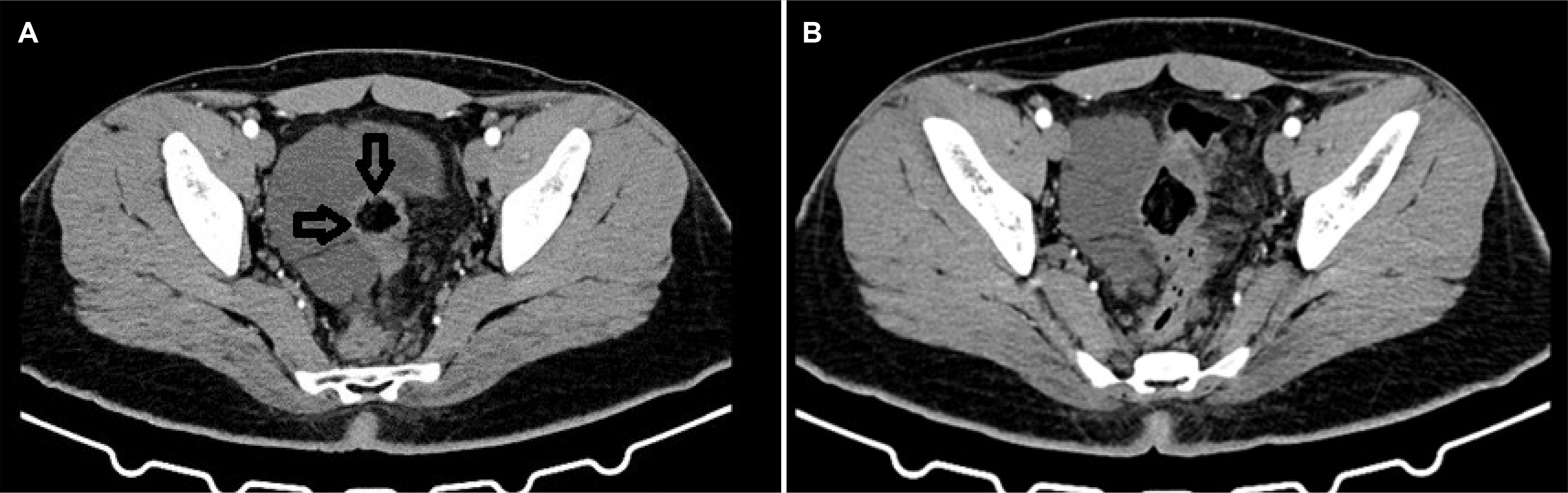 | Fig. 1Contrast enhanced computed tomography is suggestive of a multiloculated cystic lesion with well-defined enhancing walls arising from the upper rectum (axial sections). The black arrows indicate the loss of the fat plane between the lymphangioma and rectal wall (original images) 
|
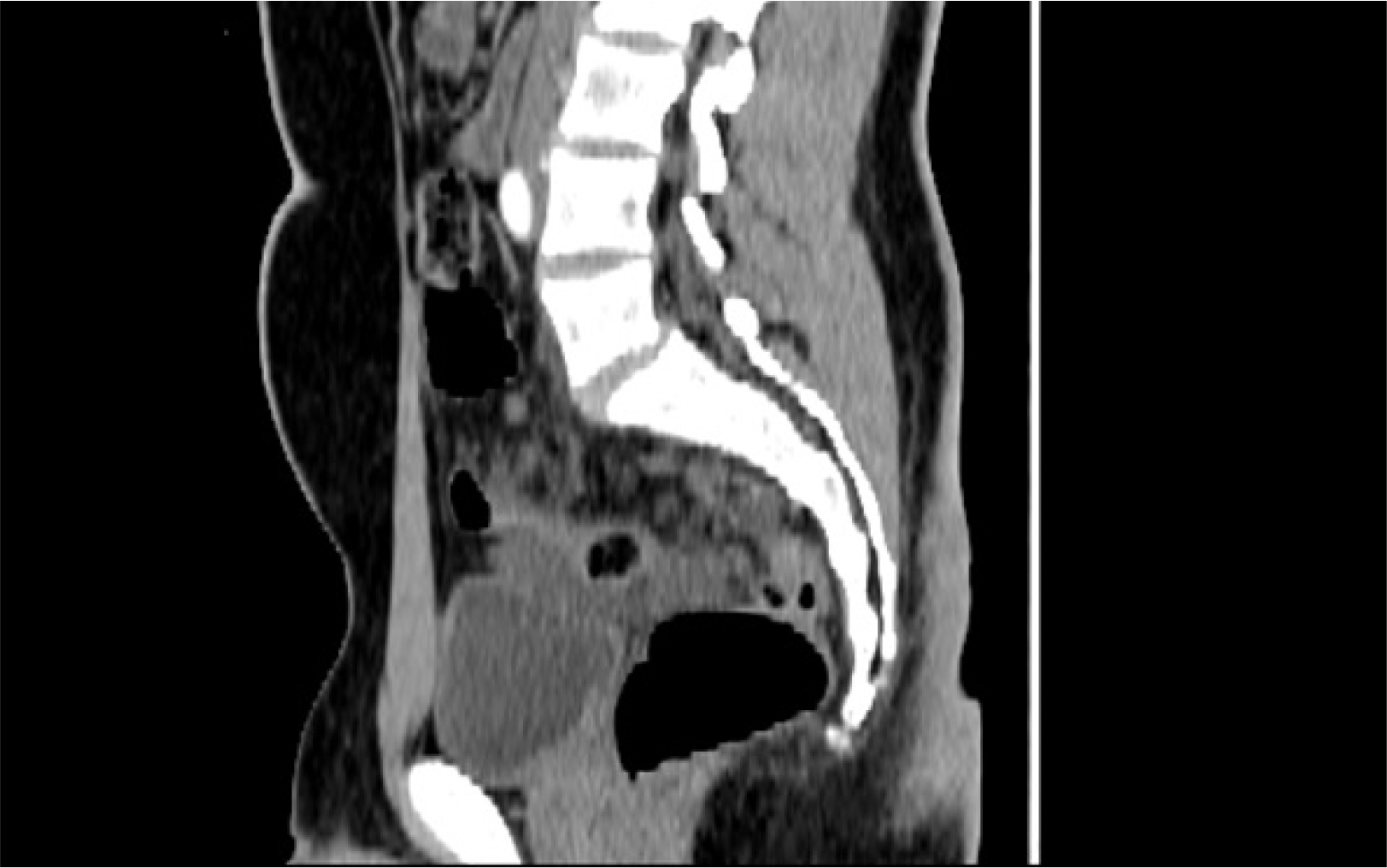 | Fig. 2Contrast enhanced computed tomography-sagittal section demonstrating the relation of lymphangioma to the urinary bladder. 
|
The colonoscopy was normal (
Fig. 3). Blood investigations did not reveal any abnormality.
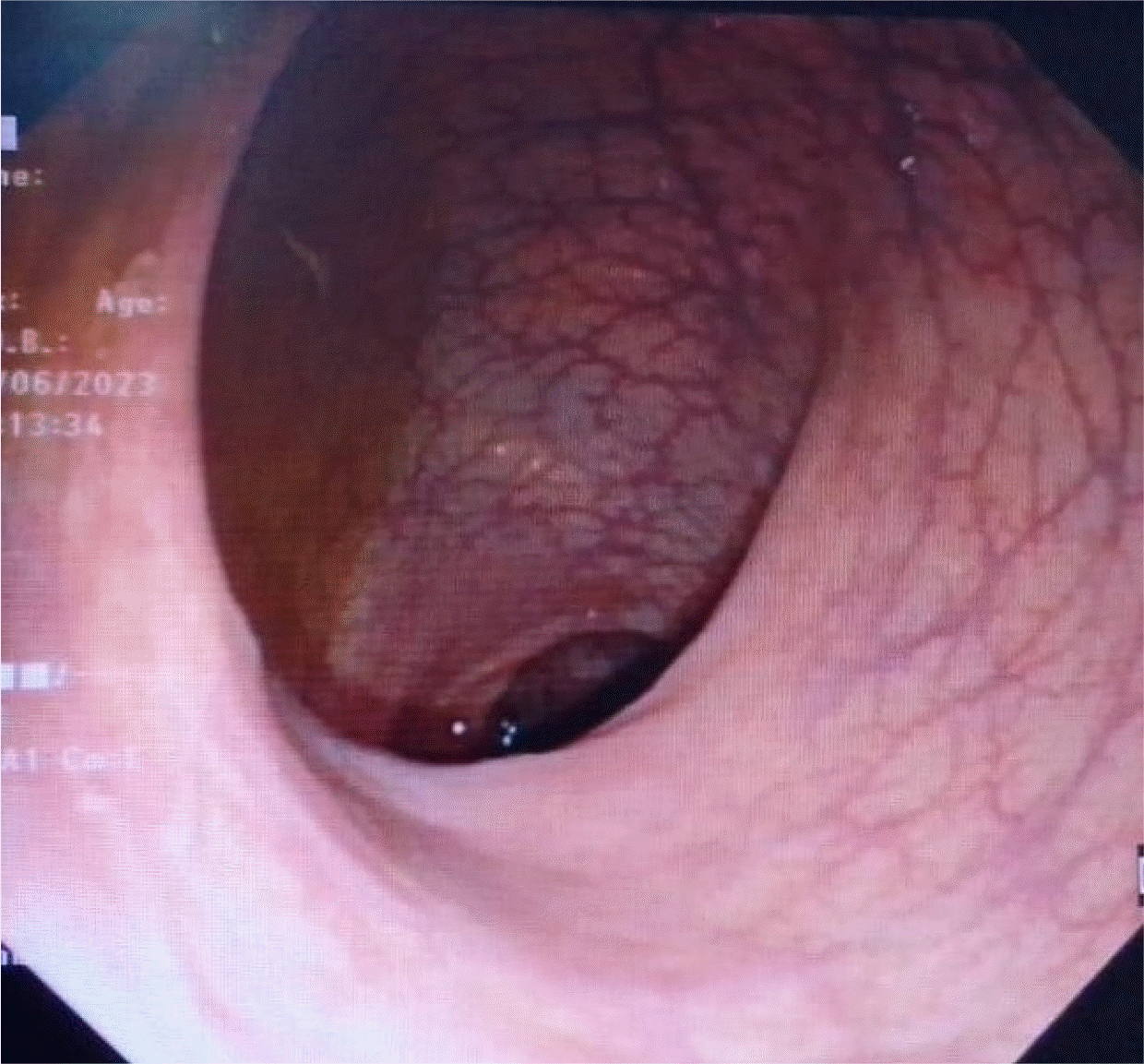 | Fig. 3
|
Informed consent was obtained, and the patient was counseled regarding the surgical intervention. He underwent open surgery through a lower midline incision. Intraoperatively, a cystic lesion of size 7×5 cm was found adhered to the upper rectal wall and occupied one-third of the circumference of the rectal wall. A low anterior resection was performed and continuity was maintained with hand-sewn colorectal anastomosis (
Fig. 4). On cutting open the gross specimen, the mucosa was normal and extraserosal extension of submucosal cystic spaces was observed (
Fig. 5). The postoperative course was uneventful, and the patient was discharged on the 6th postoperative day. Histopathology of the resected specimen confirmed it to be lymphangioma of the rectum with multiple cystic spaces in the submucosal layer (
Fig. 6). There was no evidence of malignancy. At the 1-year follow-up post-surgery, the patient was asymptomatic.
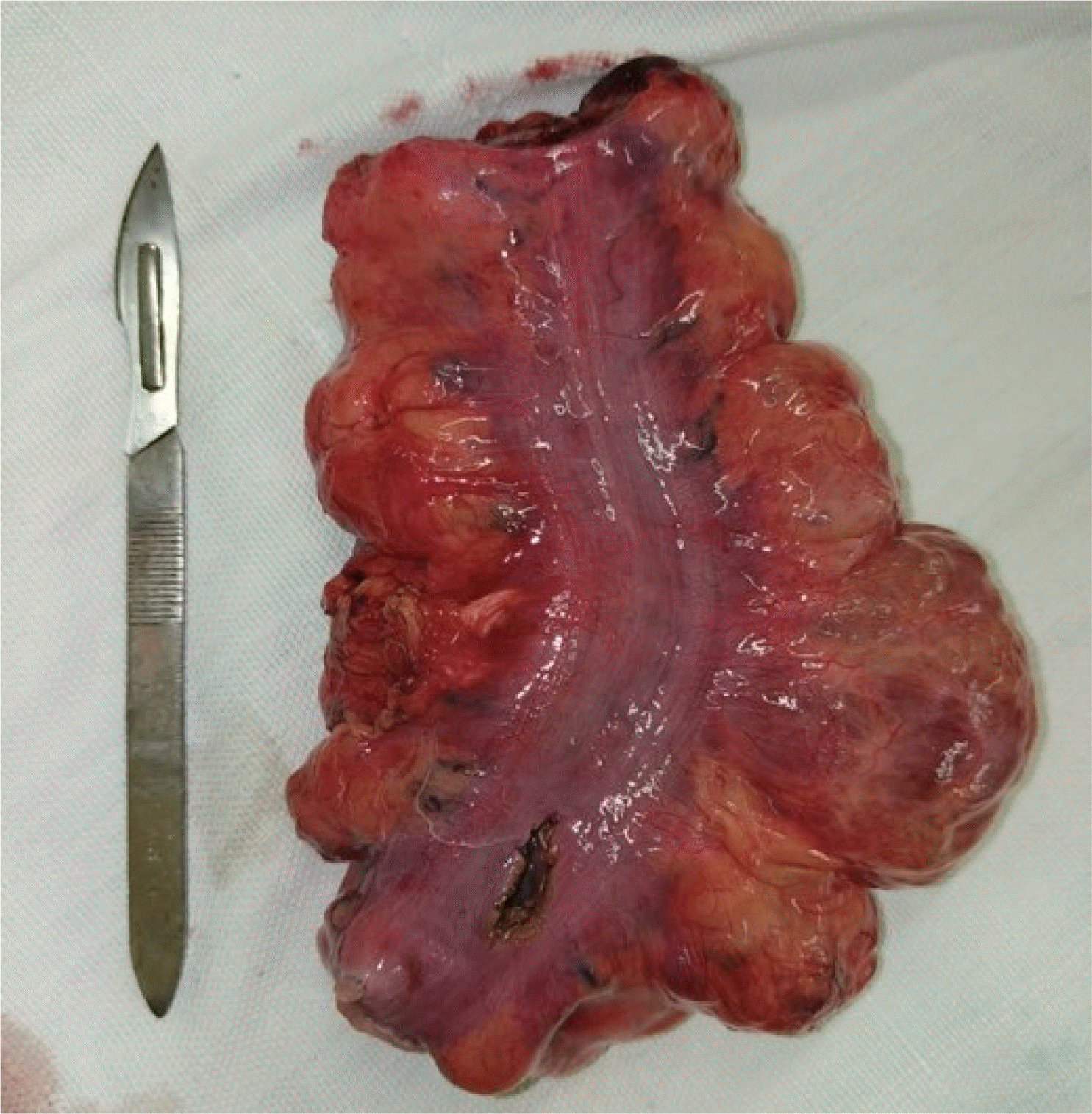 | Fig. 4Resected low anterior resection specimen with a cystic lesion along the right rectal wall (original image). 
|
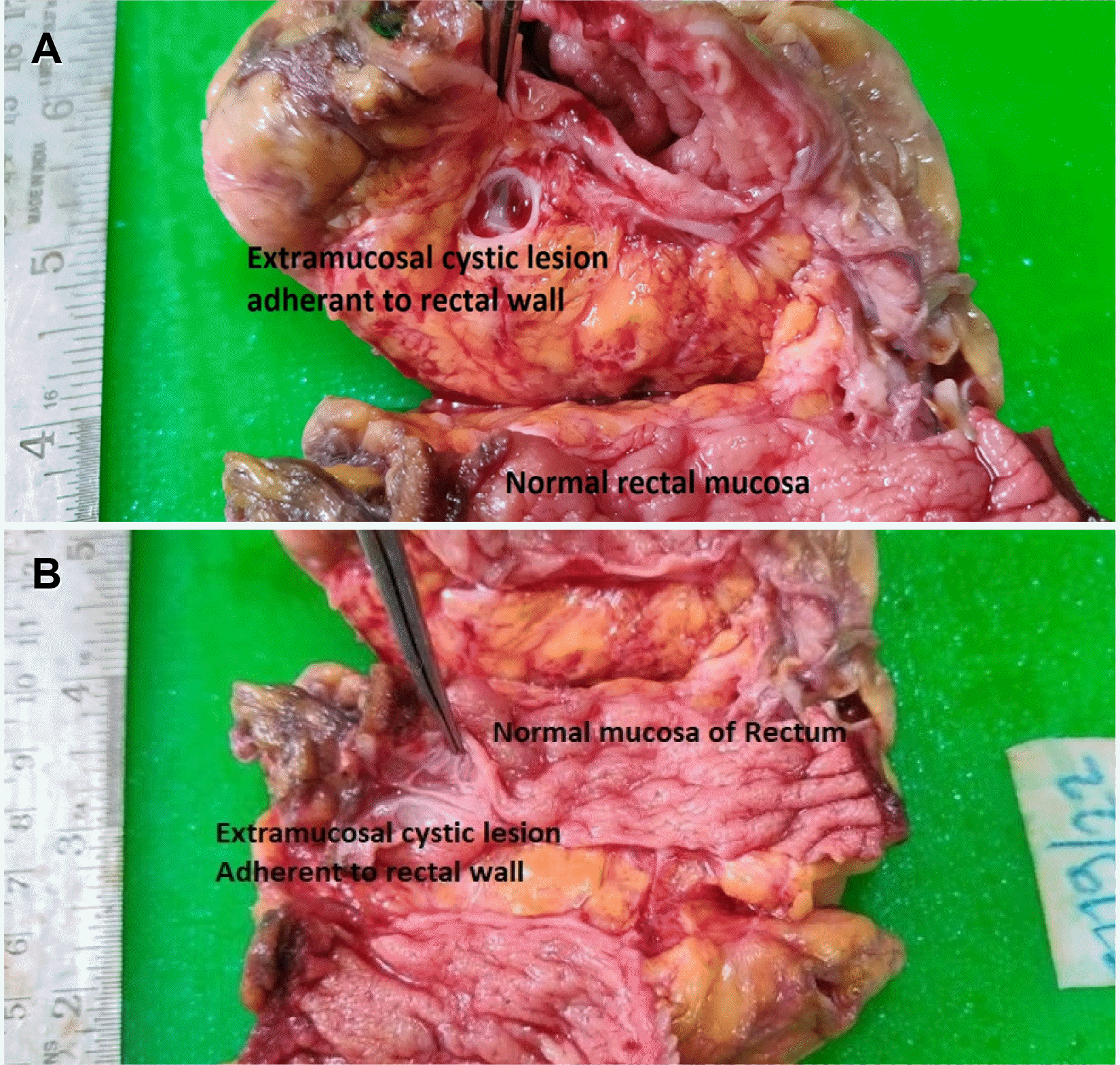 | Fig. 5Gross specimen after resection suggests normal mucosa with submucosal cystic spaces with extramural extension. 
|
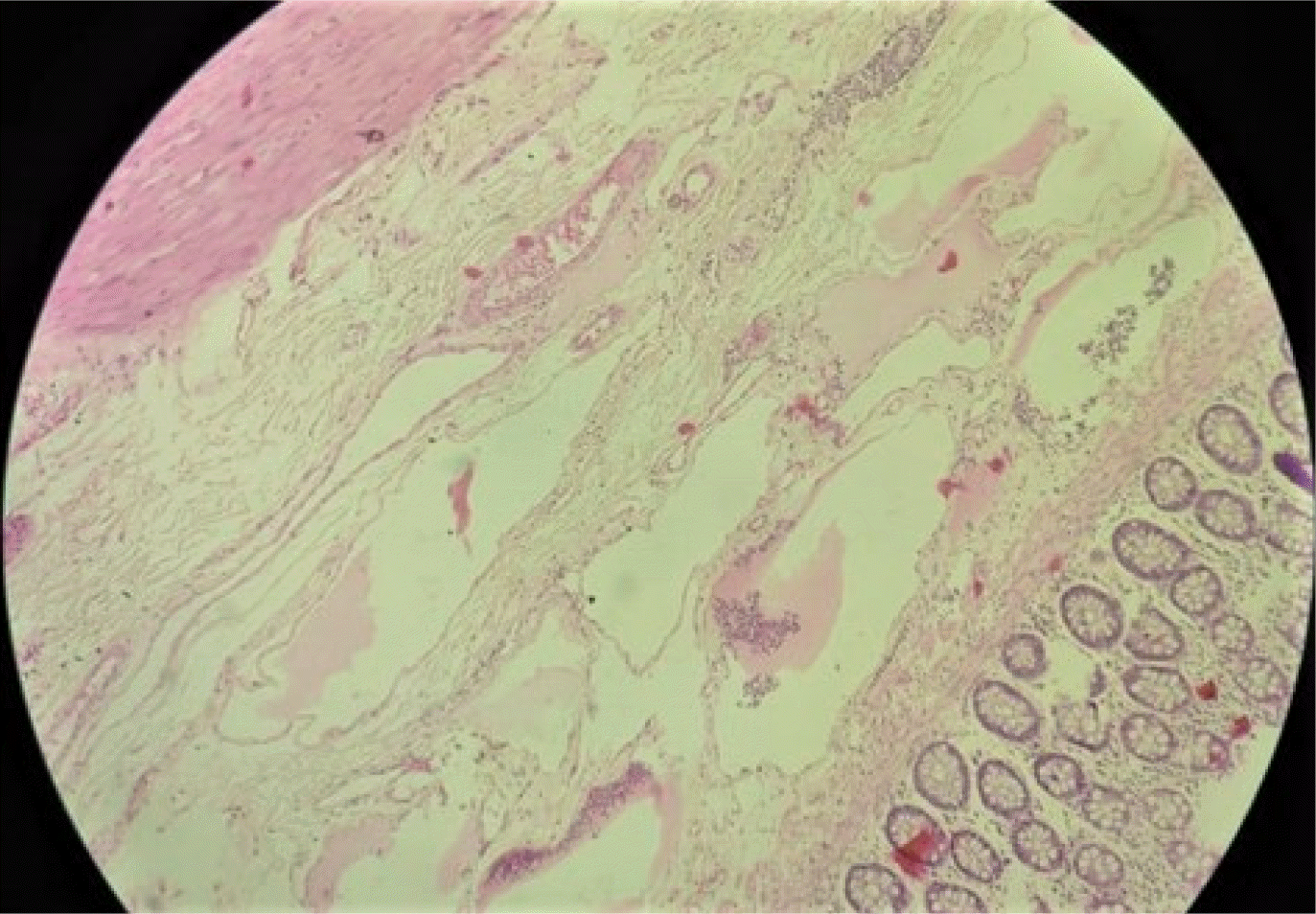 | Fig. 6Histopathological examination (HPE) suggestive of multiple cystic spaces filled with lymph lined by endothelium and separated by fibrous septae in the submucosa of the rectum with an extraserosal component. Hematoxylin and eosin (H&E) stain with 20× magnification (original image). 
|
Go to :

DISCUSSION
Colorectal lymphangioma was first reported by Chisholm in 1932.
1 In 2001, Matsuda et al.
2 reported a review of 279 cases of colorectal lymphangiomas. Of these, only 7 were of rectal origin.
2 A literature search revealed 3 more reported cases of rectal lymphangioma and 3 cases of rectal hemolymphangioma after 2001.
3-7 Colorectal lymphangioma has been often reported in East Asian patients, primarily from Japan and Korea.
2,8
Colorectal lymphangioma can occur in any age group (age range 1–83 years). It has a male preponderance (1.5:1). The most common clinical presentation is pain, abdominal discomfort, bleeding, and constipation.
2 Intestinal obstruction has not been reported. Six cases of intussusception have been reported in the literature but all were in the setting of right-sided lesions of colonic lymphangioma.
9-14 Larger lesions have been associated with massive bleeding
15 and protein- losing enteropathy.
16 Malignant transformation has not been recorded to date but malignancy may co-exist.
17
The incidence of colorectal lymphangiomas has been on the rise probably due to the increased detection with the advent of endoscopy and endoscopic ultrasound (EUS). Classically, colonic lymphangiomas are described as soft broad-based submucosal lesions with normal colonic mucosa.
18 The lesion is compressible when pushed with forceps, also called a positive cushion sign.
2 However, it may often be missed on colonoscopy if the lesion is submucosal, as it was in our case. Also, the classical colonoscopic description is seen in colonic rather than rectal lymphangiomas. The rectum has a complete circular and longitudinal muscle coat and hence, the bulging lesions described by many authors may not be apparently visible in rectal lymphangiomas. In the present case, since the lesion had predominantly extramural growth it was detected on a CECT scan.
The extraluminal extent of the lesion and its relation to the surrounding structures may be assessed on a CECT scan, wherein these masses show densitometric characteristics of the fluid type, regular margins, and only a peripheral contrast enhancement. However, the disadvantage of CECT imaging is that lesions less than 2 cm in size may be difficult to identify. In smaller lesions, endoscopic USG has shown promising results. The classical EUS findings of colonic cystic lymphangioma are submucosal anechoic cystic spaces with septations, intact muscularis propria, and no solid components.
19 In our case the CECT scan supported a diagnosis of lymphangioma.
Endoscopic approaches such as colonoscopic excision, sclerotherapy, and the use of steroids or fibrin glue have been used and have proven to be effective when the lesions are less than 2.5 cm in size. However, surgery remains the treatment for choice for larger lesions.
20
As larger lesions may produce pain, bleeding, and obstruction due to intussusception.
2,9-14 Or may compress adjacent structures, surgical treatment is preferred.
2 The lesion may sometimes co-exist with concurrent adenoma or malignancy, although the exact incidence has not been reported.
17 Recurrence has not been reported following surgical resection. Our patient underwent an open low anterior resection.
On a retrospective review of the medical records of the patient, we believe that the USG, which was done before referral to us, misinterpreted the cystic lymphangioma as a pelvic collection. Hence, the patient underwent ultrasound-guided aspiration but it recurred after an asymptomatic period of 1 year.
Morphologically lymphangiomas have been classified by Wegner
21, into three types based on the size of the lymphatic cavity and the nature of the lymphatic wall as follows: simple, cavernous, and cystic. Cystic lymphangioma is the most common type. Microscopically, lymphangioma is characterized by flat epithelial endothelium and lymphoid tissue, small lymphatic spaces, smooth muscle, and foam cells.
Several theories have been proposed as follows regarding the pathogenesis of lymphangiomas which have been reviewed by Wiegand et al.
22
1. Sequestration of lymph tissue: Sequestration of portions of primitive lymphatic anlage without connections to the lymphatic system may be attributed to the development of lymphangiomas. With infection or trauma, there is an increase in size and it becomes symptomatic.
2. Abnormal budding of lymph vessels: Some authors believe that an abnormal budding of lymphatic vessels from primitive lymphatic anlage may result in lympangiomas. They have growth potential and gradually lose drainage channels.
3. Non-fusion with the venous system: As the lymphatic system is mesenchymal in origin one of the theories of pathogenesis involves the non-fusion of the lymph sacs with the venous system. This explains the cystic hygroma in the neck where the jugulo-axillary lymph sac fails to drain into the internal jugular vein.
4. Obstruction of efferent lymph vessels: Accumulation of lymph and dilatation of lymphatic channels occurs as a result of an obstruction to the efferent vessels.
5. Genesis of lymphangiomas in adults: Small defects in the lymphatic system exist which can be compensated when the patient is healthy. Stimuli like trauma or infection trigger delayed proliferation of these cell rests.
Thus, to conclude, despite its rare occurrence rectal lymphangioma should be considered as one of the differentials in patients presenting with cystic lesions in the pelvis. These patients present with lower abdominal pain with rectal symptoms. CECT confirms the diagnosis. Normal colonoscopy does rule out the condition. Surgical resection is the preferred mode of treatment for lesions larger than 2.5 cm. as various complications are known to occur. A final diagnosis is made on histopathological examination of resected specimen which is suggestive of lesion comprising of multiple cystic spaces filled by lymph.
Go to :


