1. Cao Q, Tan CC, Xu W, Hu H, Cao XP, Dong Q, et al. The prevalence of dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2020; 73(3):1157–1166. PMID:
31884487.
2. Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet. 2013; 381(9882):2016–2023. PMID:
23746902.
3. Fiest KM, Roberts JI, Maxwell CJ, Hogan DB, Smith EE, Frolkis A, et al. The prevalence and incidence of dementia due to Alzheimer’s disease: a systematic review and meta-analysis. Can J Neurol Sci. 2016; 43(Suppl 1):S51–S82. PMID:
27307128.
4. Kim YJ, Han JW, So YS, Seo JY, Kim KY, Kim KW. Prevalence and trends of dementia in Korea: a systematic review and meta-analysis. J Korean Med Sci. 2014; 29(7):903–912. PMID:
25045221.
5. Jang JW, Park JH, Kim S, Lee SH, Lee SH, Kim YJ. Prevalence and incidence of dementia in South Korea: a nationwide analysis of the National Health Insurance Service senior cohort. J Clin Neurol. 2021; 17(2):249–256. PMID:
33835746.
6. Shin JH. Dementia epidemiology fact sheet 2022. Ann Rehabil Med. 2022; 46(2):53–59. PMID:
35508924.
7. Cheng ST. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. 2017; 19(9):64. PMID:
28795386.
8. Seok JE. Public long-term care insurance for the elderly in Korea: design, characteristics, and tasks. Soc Work Public Health. 2010; 25(2):185–209. PMID:
20391261.
9. Han JW, Jeong H, Park JY, Kim TH, Lee DY, Lee DW, et al. Effects of social supports on burden in caregivers of people with dementia. Int Psychogeriatr. 2014; 26(10):1639–1648. PMID:
25006855.
10. Shon C, Yoon H. Health-economic burden of dementia in South Korea. BMC Geriatr. 2021; 21(1):549. PMID:
34645415.
11. Porsteinsson AP, Isaacson RS, Knox S, Sabbagh MN, Rubino I. Diagnosis of early Alzheimer’s disease: clinical practice in 2021. J Prev Alzheimers Dis. 2021; 8(3):371–386. PMID:
34101796.
12. Choi H, Kim SH. Policy of national responsibility and dementia care. J Korean Med Assoc. 2018; 61(5):309.
13. Lee DW. What is needed for the success of national responsibility for dementia. J Korean Med Assoc. 2017; 60(8):618.
14. Kim SH. Future policy directions for planning of national responsibility for dementia care. J Korean Med Assoc. 2017; 60(8):622–626.
15. Cho SJ, Kang JM. Dementia incidence on the decline in Korea: lessons learned and future directions. J Korean Med Sci. 2019; 34(44):e299. PMID:
31726497.
16. Choi H, Kim SH, Lee JH, Lee AY, Park KW, Lee EA, et al. National responsibility policy for dementia care: current and future. J Korean Neurol Assoc. 2018; 36(3):152–158.
17. Petersen RC, Wiste HJ, Weigand SD, Fields JA, Geda YE, Graff-Radford J, et al. NIA-AA Alzheimer’s disease framework: clinical characterization of stages. Ann Neurol. 2021; 89(6):1145–1156. PMID:
33772866.
18. Wahlund LO, Westman E, van Westen D, Wallin A, Shams S, Cavallin L, et al. Imaging biomarkers of dementia: recommended visual rating scales with teaching cases. Insights Imaging. 2017; 8(1):79–90. PMID:
28004274.
19. Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001; 1(1):6. PMID:
11459516.
20. Xu W, Tan L, Wang HF, Tan MS, Tan L, Li JQ, et al. Education and risk of dementia: dose-response meta-analysis of prospective cohort studies. Mol Neurobiol. 2016; 53(5):3113–3123. PMID:
25983035.
21. Zhao M, Lv X, Tuerxun M, He J, Luo B, Chen W, et al. Delayed help seeking behavior in dementia care: preliminary findings from the Clinical Pathway for Alzheimer’s Disease in China (CPAD) study. Int Psychogeriatr. 2016; 28(2):211–219. PMID:
26138923.
22. Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009; 23(4):306–314. PMID:
19568149.
23. Park D, Son KJ, Jeong E, Kim H, Lee SY, Kim JH, et al. Effects of socioeconomic status and residence areas on long-term survival in patients with early-onset dementia: the Korean National Health Insurance Service Database study. J Korean Med Sci. 2022; 37(49):e354. PMID:
36536548.
24. Brayne C, Fox C, Boustani M. Dementia screening in primary care: is it time? JAMA. 2007; 298(20):2409–2411. PMID:
18042918.
25. Kaduszkiewicz H, Wiese B, van den Bussche H. Self-reported competence, attitude and approach of physicians towards patients with dementia in ambulatory care: results of a postal survey. BMC Health Serv Res. 2008; 8(1):54. PMID:
18321394.
26. Chan CQ, Lee KH, Low LL. A systematic review of health status, health seeking behaviour and healthcare utilisation of low socioeconomic status populations in urban Singapore. Int J Equity Health. 2018; 17(1):39. PMID:
29609592.
27. Kwon HS, Jeong YW, Kim SH, Park KH, Seo SW, Na HR, et al. Annual incidence of dementia from 2003 to 2018 in metropolitan Seoul, Korea: a population-based study. J Clin Med. 2022; 11(3):819. PMID:
35160270.




 PDF
PDF Citation
Citation Print
Print



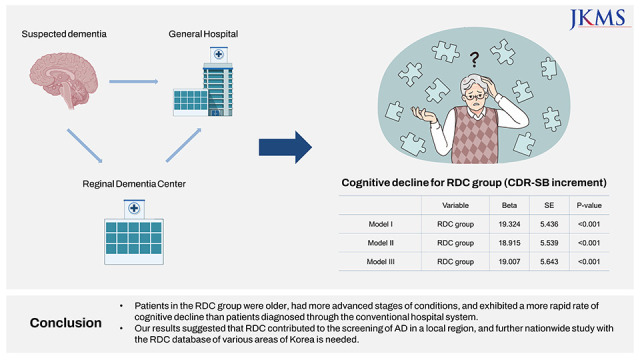
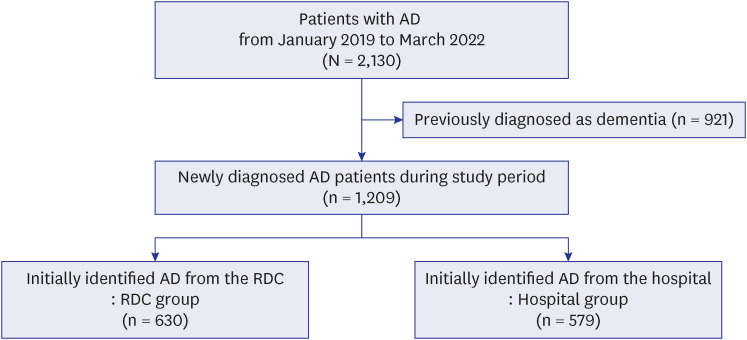
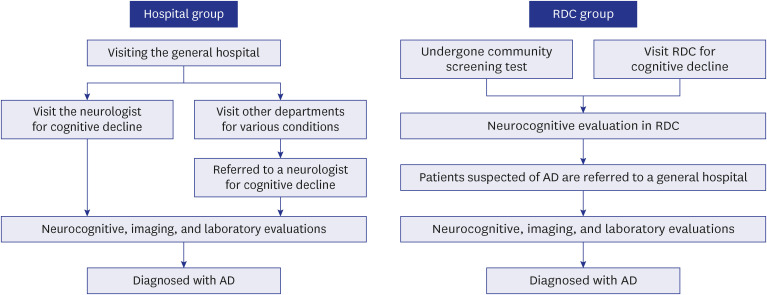
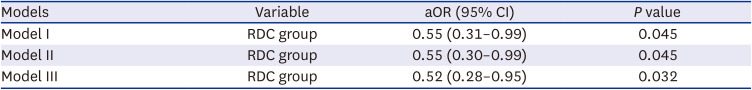
 XML Download
XML Download