1. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020; 41(3):407–477. PMID:
31504439.
2. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022; 145(3):e4–e17. PMID:
34882436.
3. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009; 360(3):213–224. PMID:
19144937.
4. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014; 371(13):1208–1217. PMID:
25176289.
5. Nørgaard BL, Fairbairn TA, Safian RD, Rabbat MG, Ko B, Jensen JM, et al. Coronary CT angiography-derived fractional flow reserve testing in patients with stable coronary artery disease: recommendations on interpretation and reporting. Radiol Cardiothorac Imaging. 2019; 1(5):e190050. PMID:
33778528.
6. Kim JE, Koo BK. Fractional flow reserve: the past, present and future. Korean Circ J. 2012; 42(7):441–446. PMID:
22870076.
7. Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (analysis of coronary blood flow using CT angiography: next steps). J Am Coll Cardiol. 2014; 63(12):1145–1155. PMID:
24486266.
8. Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011; 58(19):1989–1997. PMID:
22032711.
9. Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012; 308(12):1237–1245. PMID:
22922562.
10. Nørgaard BL, Hjort J, Gaur S, Hansson N, Bøtker HE, Leipsic J, et al. Clinical use of coronary CTA-derived FFR for decision-making in stable CAD. JACC Cardiovasc Imaging. 2017; 10(5):541–550. PMID:
27085447.
11. Jensen JM, Bøtker HE, Mathiassen ON, Grove EL, Øvrehus KA, Pedersen KB, et al. Computed tomography derived fractional flow reserve testing in stable patients with typical angina pectoris: influence on downstream rate of invasive coronary angiography. Eur Heart J Cardiovasc Imaging. 2018; 19(4):405–414. PMID:
28444153.
12. Douglas PS, De Bruyne B, Pontone G, Patel MR, Norgaard BL, Byrne RA, et al. 1-year outcomes of FFRCT-guided care in patients with suspected coronary disease: the PLATFORM study. J Am Coll Cardiol. 2016; 68(5):435–445. PMID:
27470449.
13. Yun CH, Hung CL, Wen MS, Wan YL, So A. CT assessment of myocardial perfusion and fractional flow reserve in coronary artery disease: a review of current clinical evidence and recent developments. Korean J Radiol. 2021; 22(11):1749–1763. PMID:
34431244.
14. Kwon SS, Chung EC, Park JS, Kim GT, Kim JW, Kim KH, et al. A novel patient-specific model to compute coronary fractional flow reserve. Prog Biophys Mol Biol. 2014; 116(1):48–55. PMID:
25256102.
15. Lee KE, Kwon SS, Ji YC, Shin ES, Choi JH, Kim SJ, et al. Estimation of the flow resistances exerted in coronary arteries using a vessel length-based method. Pflugers Arch. 2016; 468(8):1449–1458. PMID:
27290616.
16. Chung JH, Lee KE, Nam CW, Doh JH, Kim HI, Kwon SS, et al. Diagnostic performance of a novel method for fractional flow reserve computed from noninvasive computed tomography angiography (NOVEL-FLOW study). Am J Cardiol. 2017; 120(3):362–368. PMID:
28595860.
17. Kim SH, Kang SH, Chung WY, Yoon CH, Park SD, Nam CW, et al. Validation of the diagnostic performance of ‘HeartMedi V.1.0’, a novel CT-derived fractional flow reserve measurement, for patients with coronary artery disease: a study protocol. BMJ Open. 2020; 10(7):e037780.
18. Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014; 8(5):342–358. PMID:
25301040.
19. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013; 82(4):E266–E355. PMID:
22065485.
20. Gaur S, Achenbach S, Leipsic J, Mauri L, Bezerra HG, Jensen JM, et al. Rationale and design of the HeartFlowNXT (HeartFlow analysis of coronary blood flow using CT angiography: NeXt sTeps) study. J Cardiovasc Comput Tomogr. 2013; 7(5):279–288. PMID:
24268114.
21. Park SJ, Kang SJ, Ahn JM, Shim EB, Kim YT, Yun SC, et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC Cardiovasc Interv. 2012; 5(10):1029–1036. PMID:
23078732.
22. Tesche C, De Cecco CN, Caruso D, Baumann S, Renker M, Mangold S, et al. Coronary CT angiography derived morphological and functional quantitative plaque markers correlated with invasive fractional flow reserve for detecting hemodynamically significant stenosis. J Cardiovasc Comput Tomogr. 2016; 10(3):199–206. PMID:
26993434.
23. Wu J, Barton D, Xie F, O’Leary E, Steuter J, Pavlides G, et al. Comparison of fractional flow reserve assessment with demand stress myocardial contrast echocardiography in angiographically intermediate coronary stenoses. Circ Cardiovasc Imaging. 2016; 9(8):e004129. PMID:
27511978.
24. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44(3):837–845. PMID:
3203132.
25. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 327(8476):307–310.
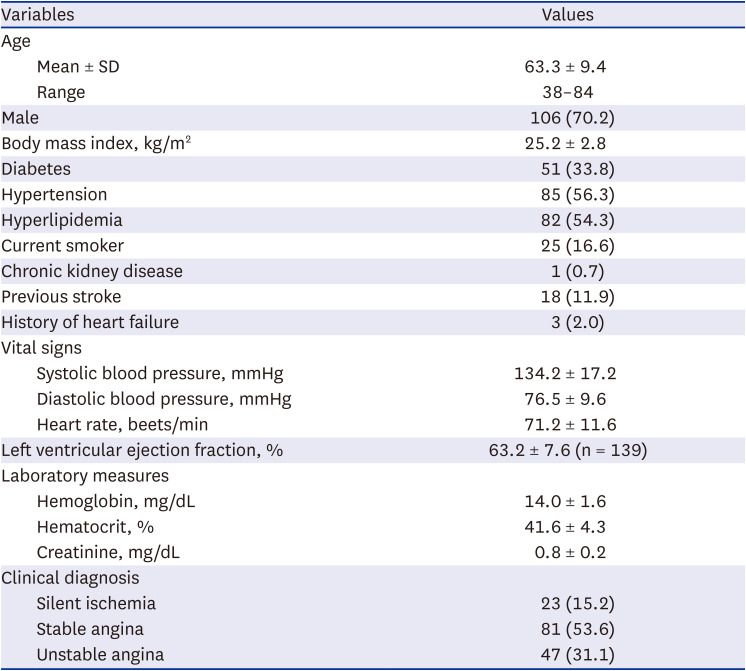
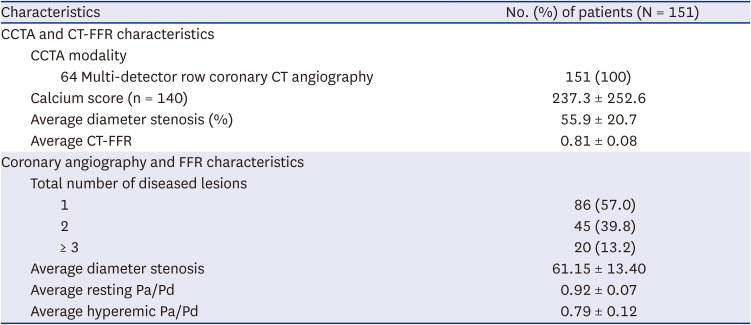
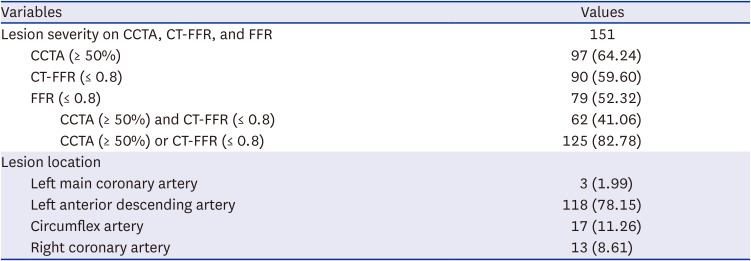
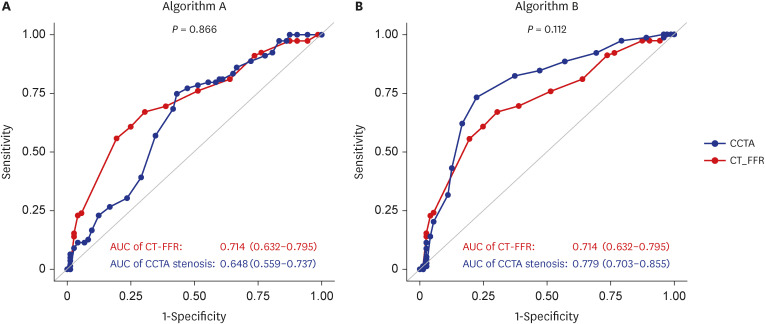




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download