1. Kizil Y, Aydil U, Ceylan A, Uslu S, Baştürk V, İleri F. Analysis of choanal polyps. J Craniofac Surg. 2014; 25(3):1082–4.
2. Lopatin A, Bykova V, Piskunov G. Choanal polyps: one entity, one surgical approach? Rhinology. 1997; 35(2):79–83.
3. Chung SK, Chang BC, Dhong HJ. Surgical, radiologic, and histologic findings of the antrochoanal polyp. Am J Rhinol. 2002; 16(2):71–6.
4. Stammberger H. Surgical treatment of nasal polyps: past, present, and future. Allergy. 1999; 54(Suppl 53):7–11.
5. Min YG, Chung JW, Shin JS, Chi JG. Histologic structure of antrochoanal polyps. Acta Otolaryngol. 1995; 115(4):543–7.
6. Cook PR, Davis WE, McDonald R, McKinsey JP. Antrochoanal polyposis: a review of 33 cases. Ear Nose Throat J. 1993; 72(6):401–11.
7. Aydin O, Keskin G, Ustündağ E, Işeri M, Ozkarakaş H. Choanal polyps: an evaluation of 53 cases. Am J Rhinol. 2007; 21(2):164–8.
8. Hong SK, Yoo YS, Shin YR, Chung SW. Choanal polyps originating from the ethmoid sinus: ethmochoanal polyps? Korean J Otorhinolaryngol-Head Neck Surg. 2002; 45(9):921–5.
9. Yaman H, Yilmaz S, Karali E, Guclu E, Ozturk O. Evaluation and management of antrochoanal polyps. Clin Exp Otorhinolaryngol. 2010; 3(2):110–4.
10. Bozzo C, Garrel R, Meloni F, Stomeo F, Crampette L. Endoscopic treatment of antrochoanal polyps. Eur Arch Otorhinolaryngol. 2007; 264(2):145–50.
11. Suzuki S, Yasunaga H, Matsui H, Fushimi K, Kondo K, Yamasoba T. Complication rates after functional endoscopic sinus surgery: analysis of 50,734 Japanese patients. Laryngoscope. 2015; 125(8):1785–91.
12. We J, Lee WH, Tan KL, Wee JH, Rhee CS, Lee CH, et al. Prevalence of nasal polyps and its risk factors: Korean National Health and Nutrition Examination Survey 2009-2011. Am J Rhinol Allergy. 2015; 29(1):e24–8.
13. Fahy C, Jones NS. Nasal polyposis and facial pain. Clin Otolaryngol Allied Sci. 2001; 26(6):510–3.
14. Youn EK, Chung EC, Lee YU. CT and MR evaluation of choanal polyps. J Korean Radiol Soc. 1998; 39(2):283–8.
15. De Vuysere S, Hermans R, Marchal G. Sinochoanal polyp and its variant, the angiomatous polyp: MRI findings. Eur Radiol. 2001; 11(1):55–8.
16. Diamantopoulos II, Jones NS, Lowe J. All nasal polyps need histological examination: an audit-based appraisal of clinical practice. J Laryngol Otol. 2000; 114(10):755–9.
17. Kim SJ, Lee KH, Kim SW, Cho JS, Park YK, Shin SY. Changes in histological features of nasal polyps in a Korean population over a 17-year period. Otolaryngol Head Neck Surg. 2013; 149(3):431–7.
18. Berg O, Carenfelt C, Silfverswärd C, Sobin A. Origin of the choanal polyp. Arch Otolaryngol Head Neck Surg. 1988; 114(11):1270–1.
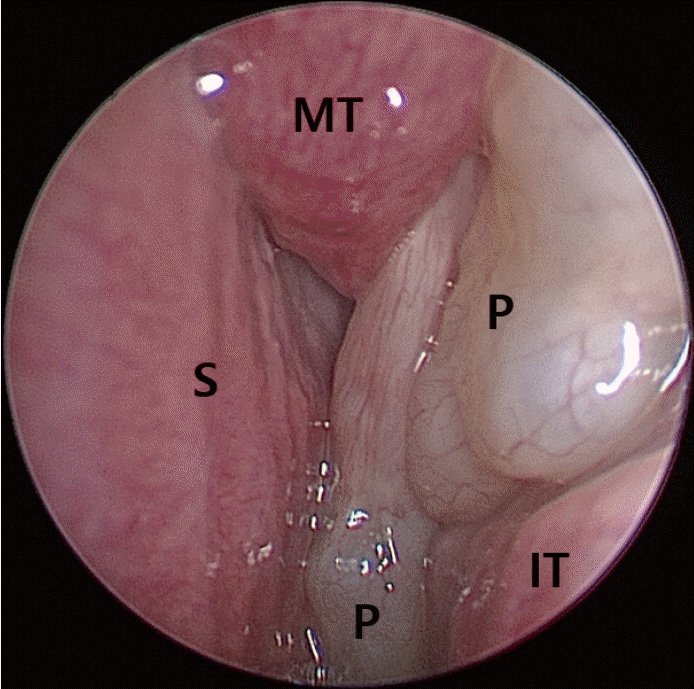
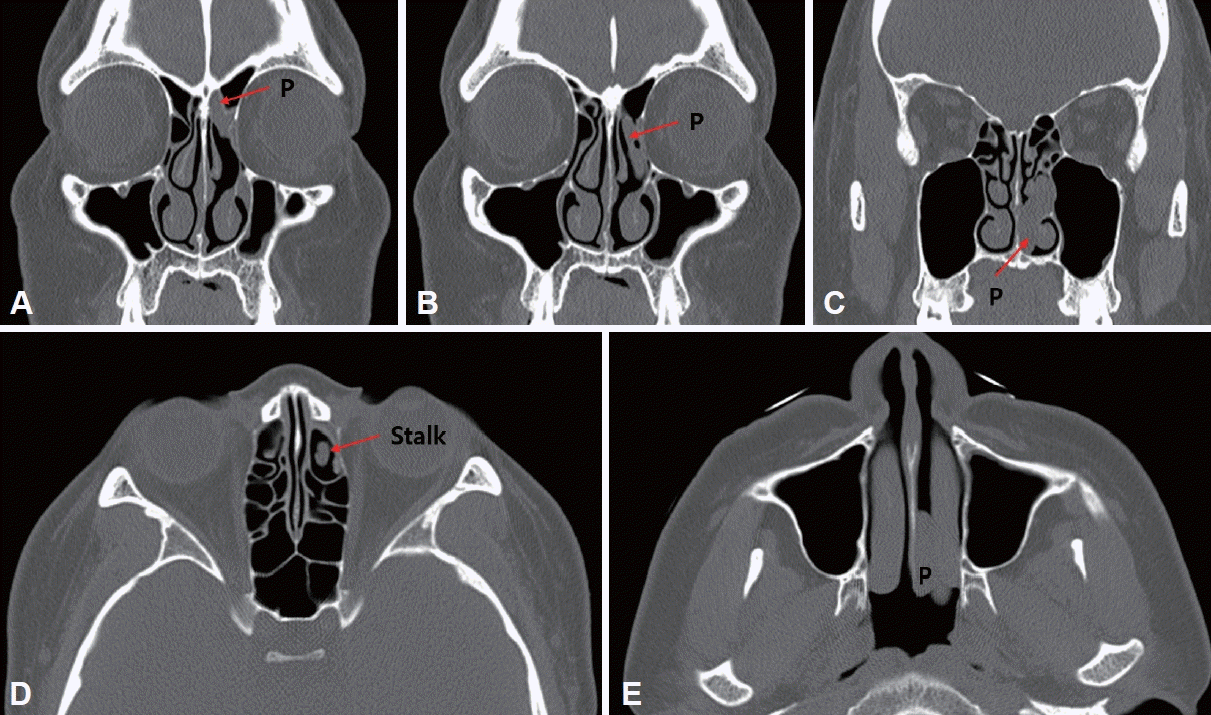
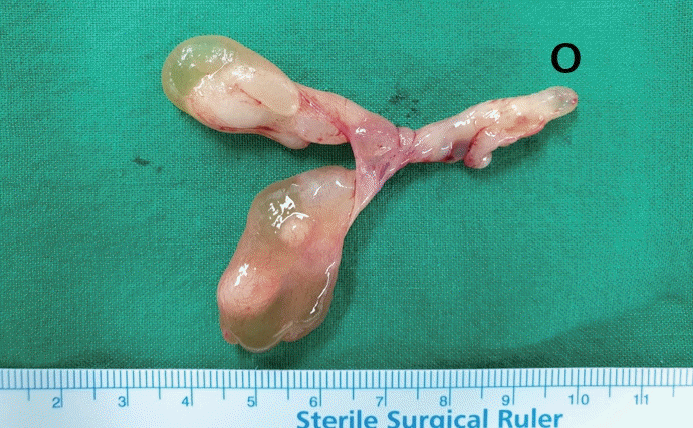
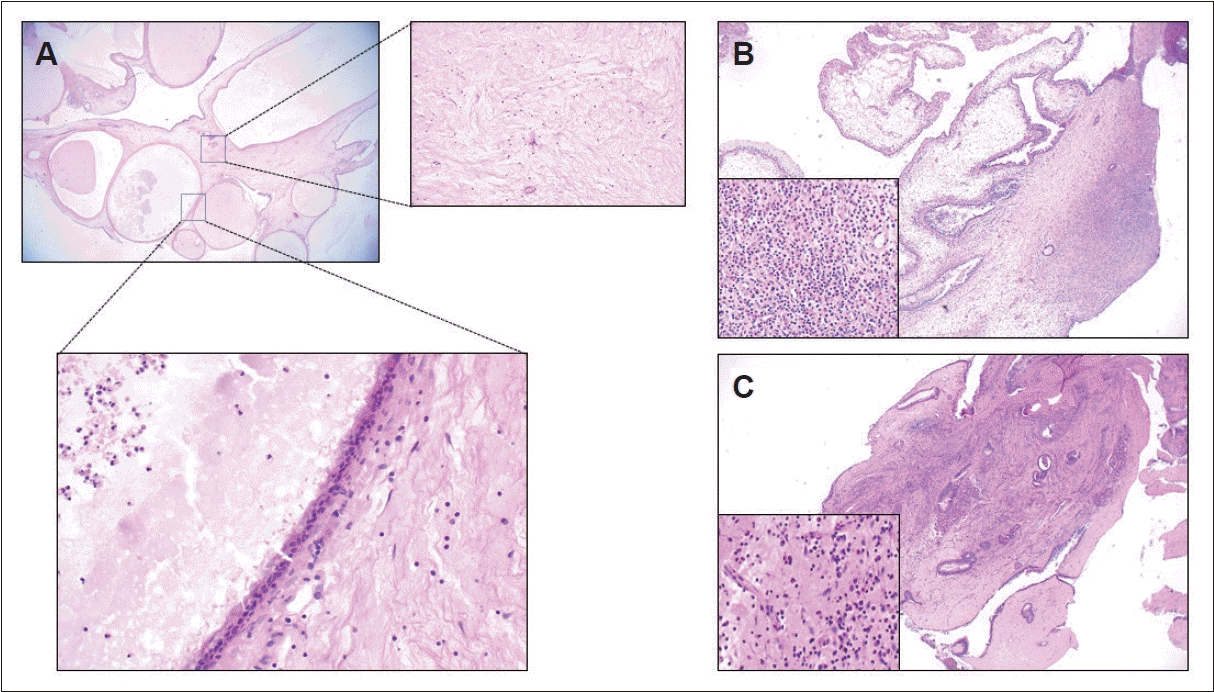




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download