Abstract
Objective
To measure muscle activity before and after robot-assisted gait training (RAGT) in patients with stroke and examine the differences in muscle activity changes compared with conventional gait training (CGT).
Methods
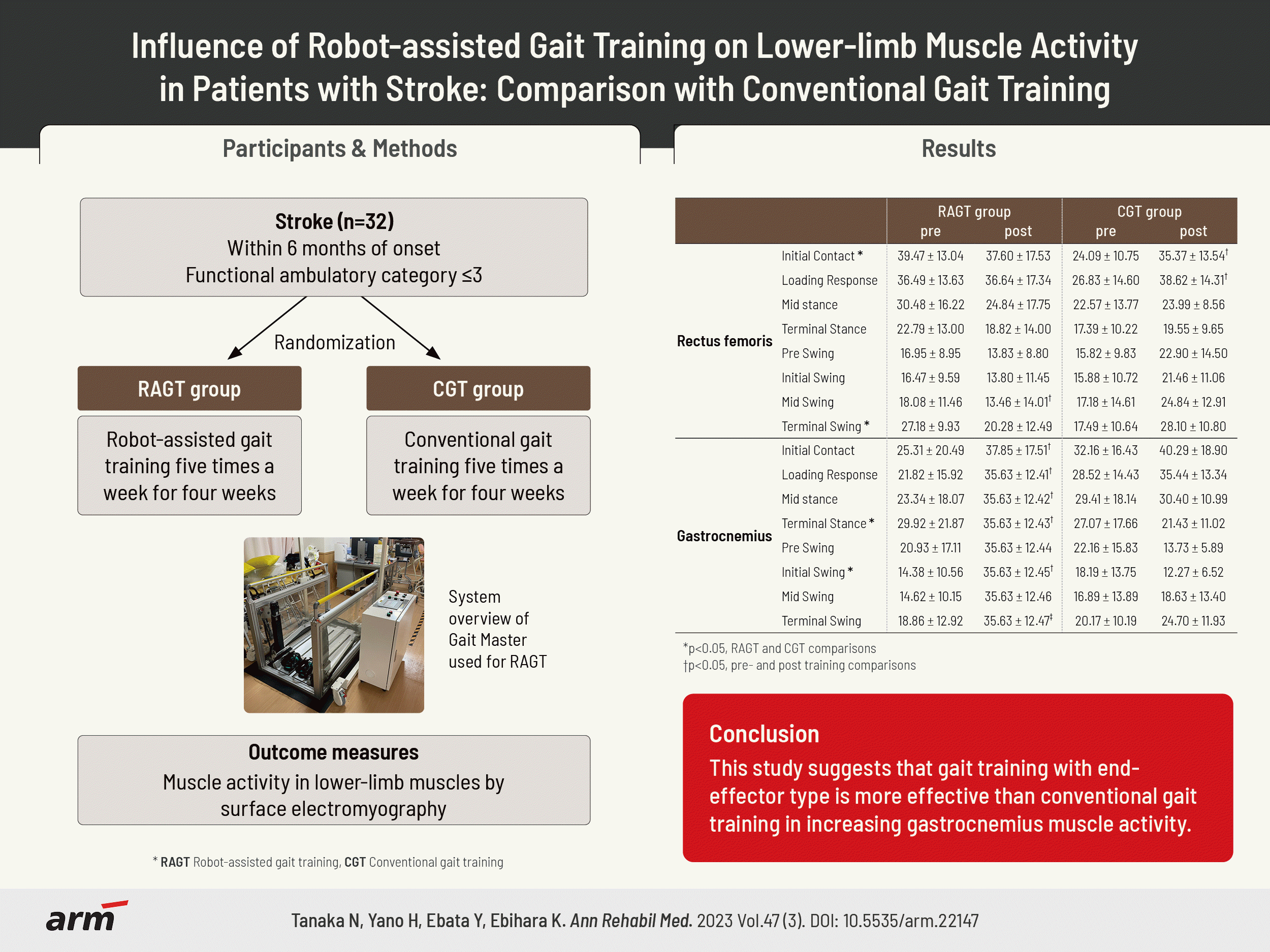
Thirty patients with stroke (RAGT group, n=17; CGT group, n=13) participated in the study. All patients underwent RAGT using a footpad locomotion interface or CGT for 20 minutes for a total of 20 sessions. Outcome measures were lower-limb muscle activity and gait speed. Measurements were performed before the start of the intervention and after the end of the 4-week intervention.
Results
The RAGT group showed increased muscle activity in the gastrocnemius, whereas the CGT group showed high muscle activity in the rectus femoris. In the terminal stance of the gait cycle, the gastrocnemius, the increase in muscle activity was significantly higher in the RAGT group than in the CGT group.
Stroke is well known to significantly impair motor and sensory functions, and the resulting gait disturbance persists even after three months [1]. Therefore, restoring gait ability is the most critical task in post-stroke rehabilitation [2].
In recent years, gait rehabilitation using robot-assisted gait training (RAGT) has been the focus of attention in gait recovery after a stroke. RAGT is a method of training a patient’s lower limbs to move in a gait-like movement using actuators attached to a walking device [3]. RAGT involves the actuator performing the movements of the patient’s lower limbs during walking, thus enabling intensive and repetitive gait practice necessary to relearn gait [4].
RAGT can be classified into exoskeletal and end-effector types according to how the lower limbs move [5], such as LOKOMAT (Hocoma AG, Volketswil, Switzerland) [6-10] and GaitMaster [11-14], respectively. In a previous report on RAGT for patients with stroke, Schwartz et al. [15] performed RAGT with LOKOMAT in patients with subacute stroke. They showed that it was effective in restoring gait ability compared with usual physiotherapy. Tanaka et al. [16] also reported that gait rehabilitation using GaitMaster for patients with subacute stroke resulted in significantly higher gait speed and endurance improvement than conventional gait rehabilitation. Thus, the benefits of RAGT for patients with stroke are promising [17-20]; however, some reports have found no significant difference when compared with conventional gait rehabilitation [21-23]. Therefore, neurophysiological assessment is needed to further understand the effects of RAGT [24].
The neurophysiological effects of RAGT have been studied in lower-limb muscle activity. Hidler and Wall [25] measured muscle activity during RAGT with LOKOMAT and treadmill walking in healthy participants. They reported higher muscle activity in the rectus femoris and vastus lateralis during the free leg phase in RAGT because of pelvic restriction and lower activity in the gastrocnemius and tibialis anterior muscles during the overall walking cycle as a result of passive walking in RAGT. Coenen et al. [26] also measured lower-limb muscle activity during RAGT with LOKOMAT and overground walking in patients with chronic stroke and reported that lower-limb muscle activity in these patients was lower during RAGT than during overground walking. These studies have shown that RAGT reduces lower-limb muscle activity. However, reports so far have not shown how continuous training of RAGT changes muscle activity in patients with stroke. Thus, further studies are needed to assess changes in muscle activity before and after RAGT to improve understanding of the effects of RAGT on muscle activity.
Therefore, this study aimed to measure changes in muscle activity pre- and post-RAGT in patients with stroke and investigate the differences between conventional gait training (CGT) and changes in muscle activity. Concretely, we hypothesized that RAGT with an end-effector-type device would induce ankle joint muscle activity and positively change the ankle muscle activity pattern more than CGT.
Patients with stroke within 6 months of onset and significant gait disorder (functional ambulatory category [27] of ≤3) on study entry were included. All participants were inpatients. Patients who could not walk independently before onset, patients with severe cardiovascular or pulmonary dysfunction (equivalent to New York Heart Association Classification [28] III or IV), patients with osteoarthritic disease limiting movement, and patients with dementia and difficulty understanding instructions (mini-mental state examination [29] <20) were excluded.
The Ethics Committee of the Tokyo Professional University of Health Sciences approved the study (Approval No. 21-0024). This study was conducted with the consent of all participants or their legal representatives to participate in the study.
The participants were assigned to the RAGT group (odd numbers) or CGT (even numbers) using a computer-generated random number table. In the RAGT group, RAGT using a footpad-type locomotion interface was performed five times a week for 4 weeks, and CGT was not performed. The footpad-type locomotion interface used in this study was GaitMaster, which was developed by the Division of Intelligent Interaction Technologies, Faculty of Engineering, Information and Systems, University of Tsukuba [30]. GaitMaster is an end-effector type gait support device that can present gait-like movements by combining back-and-forth and up-and-down movements of the footpads. The participants place their legs on the footpads, and the footpads move along the gait trajectory, enabling the user to perform a gait-like movement [31].
The gait training conditions using the GaitMaster were as follows: the walking speed was the maximum walking speed possible for the participant to perform the gait movement, and the training time was 20 minutes, including rest time. A safety belt was attached to the weight-loading device during gait training to ensure safety, but no weight-unloading was performed. In addition, participants used handrails attached to the GaitMaster as necessary to ensure safety. Patients belonging to RAGT group did not use foot braces during training.
In the CGT group, participants performed CGT to acquire independent gait five times a week for 4 weeks. CGT consisted of stepping exercises, parallel bars, and walking exercises on the floor. The CGT group did not use treadmills or other commonly used gait practice equipment. The physiotherapists modified the content of the gait training for the CGT group according to the patient’s ability and condition. Additionally, the physiotherapist provided gait training in the CGT group using a metal-upright ankle–foot orthosis (n=7), a shoehorn-type ankle–foot orthosis (n=1), and an off-the-shelf soft knee brace (n=1), depending on the patient’s gait ability. The gait training time for the CGT group was 20 min, including rest periods. Neither the RAGT nor the CGT group had any restrictions on rehabilitation other than walking exercises, such as occupational therapy or speech and language therapy.
Participants’ age, sex, time since onset, stroke type, paralysis side, lower-extremity Fugl–Meyer assessment (LE-FMA), functional ambulation category (FAC), gait speed, and lower-limb muscle strength were baseline assessments.
The primary outcome measure was muscle activity in lower-limb muscles by surface electromyography. The electromyogram (EMG) was measured using the wireless electromyography sensor SS-WS2911 (Sports Sensing; Fukuoka, Japan). Gluteus medius, rectus femoris, hamstrings, tibialis anterior, and gastrocnemius muscles on the paralyzed side were evaluated. The electrode attachment positions for each muscle followed the surface EMG for non-invasive assessment of muscles guidelines [32]. A foot switch was also affixed to the heel to measure pressure data.EMG measurements were performed with floor walking. The participant’s muscle activity for ten walking cycles was recorded at a sampling frequency of 1,000 Hz. The acquired data were processed with a bandpass filter at 10–500 Hz and smoothed by root mean square (RMS) every 100 ms. The RMS-processed waveforms were normalized to 100% of the maximum myopotential for each muscle. The normalized ten walking cycles of the waveform data were converted to one walking cycle (0%–100%) by additive averaging using footswitch data. The converted data for one gait cycle was divided into the initial contact phase (0%–1%), loading response phase (2%–12%), mid-stance phase (13%–31%), terminal stance phase (32%–50%), pre-swing phase (51%–62%), initial swing phase (63%–75%), mid-swing phase (76%–87%), and terminal swing phase (88%–100%) [33].
The secondary assessment outcome was the gait speed. Gait speed was defined as the maximum walking speed in floor walking. Gait speed was measured by having the participants walk as fast as possible on a 10-m walking path and calculating their walking speed using the number of seconds required to do the walk. Two measurements were made for the gait speed. The faster gait speed was adopted as the maximum gait speed. When measuring walking speed, the use of common walking aids was permitted, and walking assistance by a physiotherapist was allowed if necessary. These assessments were measured before the study began and at the end.
Comparisons between the RAGT and CGT groups for age, time since onset, LE-FMA, FAC, gait velocity, and lower-limb muscle strength at the baseline were made using an unpaired t-test for those following a normal distribution after conducting the Shapiro–Wilk test or the Mann–Whitney U-test for those not following a normal distribution. Comparisons were made for sex, stroke type, and paralyzed side using Fisher’s direct significant difference establishment.
Pre- and post-comparisons of gait speed and muscle activity in each group were made using the Shapiro–Wilk test followed by a paired t-test for those following a normal distribution and the Wilcoxon signed rank test for those not following a normal distribution. The changes before and after the intervention were calculated for between-group comparisons of walking speed and muscle activity. After performing the Shapiro–Wilk test, those following a normal distribution were compared using an unpaired t-test, and those not following a normal distribution were compared using the Mann–Whitney U-test. All statistical analyses were performed using IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, NY, USA), with a statistical significance set at 0.05.
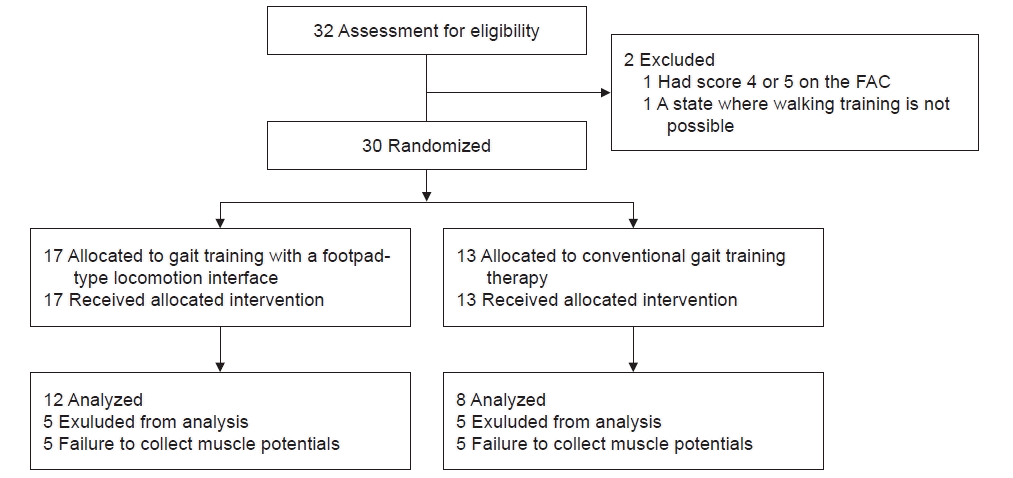
We enrolled 32 participants in this study; however, 1 participant having FAC of ≥3 and 1 participant who could not perform gait training were excluded from the study, leaving a total of 30 participants. The enrolled participants were divided into RAGT groups (n=17) for odd numbers and CGT groups (n=13) for even numbers, using a computer-generated random number table. All participants completed the study. However, five participants in the RGAT group and five in the CGT group were excluded from the analysis because their muscle
Gait speed increased significantly in both groups (RAGT: pre, 0.45±0.28 m/s vs. post, 0.73±0.55 m/s; p=0.027; CGT: pre, 0.28±0.25 m/s vs. post, 0.56±0.47 m/s; p=0.017).
Regarding muscle activity changes within the groups, the RAGT group showed a significant increase in muscle activity in the gastrocnemius throughout the gait cycle. In the CGT group, a significant increase in muscle activity was found in the early stance phase in the rectus femoris (Table 2).
The change in the walking speed between the RGAT and CGT groups before and after training showed no significant differences between the two groups (RAGT, 0.27±0.37 m/s vs. CGT, 0.28±0.26 m/s; p=0.337).
A comparison of muscle activity between the RAGT and CGT groups showed an increase in the muscle activity in the CGT group at the initial contact and terminal swing in the rectus femoris and a higher increase in muscle activity in the RAGT group at the terminal stance and an initial swing in the gastrocnemius (Table 2).
This study compared the changes in lower-limb muscle activity between gait rehabilitation with RAGT using GaitMaster and CGT in patients with stroke and gait disorders. To the best of our knowledge, no longitudinal study has examined changes in muscle activity following RAGT-based interventions. This study is probably the first study in that category. In the RAGT group, a significant increase in muscle activity in the gastrocnemius muscles was found, particularly in the terminal stance phase, after 4 weeks of RAGT intervention with GaitMaster. By contrast, after 4 weeks of intervention, the CGT group showed a significant increase in muscle activity in the early stance phase of the rectus femoris.
In a previous study of the effects of RAGT on muscle activity, Hidler and Wall [25] reported that muscle activity during walking with an exoskeletal LOKOMAT was generally higher in the quadriceps and gluteus maximus than during treadmill walking but lower in the gastrocnemius, long adductor, and tibialis anterior. The results of the present study were inconsistent with those of previous studies. The disagreement is probably due to differences in the induction method of the skeletal and end-effector lower limbs. RAGT with GaitMaster differs from CGT in that the ankle joint is in a gait position with the ankle joint fixed to the footpad [31]. The plantar surface of the foot always touches the footpad, which means that the lower-limb is loaded proportionately, even during the swing phase. In addition, RAGT with GaitMaster can induce the terminal stance phase of the lower-limb on the stance side through the backward movement of the footpad. Compared with CGT, this combined effect may have led to an increase in gastrocnemius muscle activity throughout the gait cycle, particularly in the terminal stance phase. In contrast, CGT significantly increased muscle activity in the early stance phase of the rectus femoris. As Prosser et al. [34] reported similar muscle activity patterns for ground and treadmill walking, so we consider that muscle activity during treadmill walking can be used as a reference. Regarding treadmill walking in stroke patients, van Kammen et al. [35] reported higher muscle activity in the lateral vastus muscles during the early stance phase of treadmill walking compared with LOKOMAT and walking. Repeated CGT may have increased the muscle activity of the rectus femoris in the early stance phase. However, as described by Semaan et al. [36], the relationship between CGT and increased muscle activity in the rectus femoris should be further investigated, as treadmill walking and ground walking has similar muscle activity patterns differ in the amplitude of muscle activity and kinematic measures. Moreover, the CGT group used foot braces during gait training. Further investigation into the relationship between foot braces and rectus femoris muscle activity is needed, as ankle joint immobilization with foot braces may have increased stability during the stance phase and influenced the activity of the rectus femoris muscles.
Post-stroke gait disorders are affected by reduced propulsion from the paralyzed side [37], leading to reduced walking speed and asymmetry [38]. Therefore, propulsion improvement on the paralyzed side may be necessary for recovery from gait disorders after a stroke [39]. The gastrocnemius muscles mainly generate the propulsive force of walking. Therefore, it is crucial to improve the strength of the gastrocnemius. Varoqui et al. [40] reported that repetitive gait movements with the LOKOMAT did not worsen the plantar flexor ankle muscles’ muscle tone but improved their strength. Therefore, here, the increase in gait speed of the RAGT group in this study can be attributed to increased muscle activity in the gastrocnemius due to repetitive gait movements using the GaitMaster, which increases muscle strength. However, this study did not assess ankle plantar flexion muscle strength and gastrocnemius muscle tone. These factors should be assessed in future studies. Additionally, as this study only assessed the paralyzed side, future research on improving symmetry is required.
We observed no significant difference in the gait speed improvement between the RAGT and CGT groups. However, a previous report by Tanaka et al. [16] found that RAGT with GaitMaster had greater gait speed improvement than that in the CGT group. In this study, lower-limb muscle strength in the CGT group tended to be higher than that in the RAGT group at the beginning of the study, although this difference was not significant. However, it is possible that this difference in muscle strength could have influenced the increase in gait speed.
This study found that RAGT and CGT elicit muscle activities in different regions. This finding indicates that RAGT and CGT have different mechanisms of gait disorder recovery. Many previous RAGT studies have focused on improving gait ability; however, very few have focused on the mechanism of gait recovery. Therefore, future studies should examine the effects of RAGT from the aspect of gait recovery mechanisms.
This study has several limitations. First, the statistical power was low because of the small number of participants and analysis because of errors in EMG measurements. Second, the results are limited to RAGT using GaitMaster and therefore cannot be generalized to all robot gait devices, as many robot gait device types perform RAGT, and the mechanism for performing RAGT differs from one type to another. Therefore, future studies should generalize the effects of RAGT on muscle activity by increasing the number of participants and identifying differences in the results on muscle activity among robotic gait devices.
In conclusion, the results suggest that RAGT with GaitMaster is more effective than CGT in increasing muscle activity in the gastrocnemius muscle, which is involved in the propulsive force of the gait.
ACKNOWLEDGMENTS
We thank the patients who participated in this study. In addition, we are very grateful to the rehabilitation staff at Hitachinaka General Hospital for their cooperation in data collection.
This study was supported by JSPS KAKENHI (Grant-in-Aid for Young Scientists [B]: 16K16459).
Notes
Conceptualization: Tanaka N, Yano H. Methodology: Tanaka N, Ebihara K, Ebata Y. Formal analysis: Tanaka N. Funding acquisition: Tanaka N. Project administration: Tanaka N, Yano H. Visualization: Tanaka N, Ebihara K, Writing – original draft: Tanaka N, Ebihara K, Ebata Y. Writing – review and editing: Tanaka N. Approval of the final manuscript: all authors.
REFERENCES
2. Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med. 2005; 352:1677–84.
3. Hesse S, Uhlenbrock D. A mechanized gait trainer for restoration of gait. J Rehabil Res Dev. 2000; 37:701–8.
4. Cumming TB, Thrift AG, Collier JM, Churilov L, Dewey HM, Donnan GA, et al. Very early mobilization after stroke fast-tracks return to walking: further results from the phase II AVERT randomized controlled trial. Stroke. 2011; 42:153–8.

5. Buesing C, Fisch G, O'Donnell M, Shahidi I, Thomas L, Mummidisetty CK, et al. Effects of a wearable exoskeleton stride management assist system (SMA®) on spatiotemporal gait characteristics in individuals after stroke: a randomized controlled trial. J Neuroeng Rehabil. 2015; 12:69.

6. Colombo G, Wirz M, Dietz V. Driven gait orthosis for improvement of locomotor training in paraplegic patients. Spinal Cord. 2001; 39:252–5.

7. Jezernik S, Colombo G, Keller T, Frueh H, Morari M. Robotic orthosis lokomat: a rehabilitation and research tool. Neuromodulation. 2003; 6:108–15.

8. Husemann B, Müller F, Krewer C, Heller S, Koenig E. Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after stroke: a randomized controlled pilot study. Stroke. 2007; 38:349–54.

9. Mayr A, Kofler M, Quirbach E, Matzak H, Fröhlich K, Saltuari L. Prospective, blinded, randomized crossover study of gait rehabilitation in stroke patients using the Lokomat gait orthosis. Neurorehabil Neural Repair. 2007; 21:307–14.

10. van Nunen MP, Gerrits KH, Konijnenbelt M, Janssen TW, de Haan A. Recovery of walking ability using a robotic device in subacute stroke patients: a randomized controlled study. Disabil Rehabil Assist Technol. 2015; 10:141–8.

11. Yano H, Kasai K, Saitou H, Iwata H. Development of a gait rehabilitation system using a locomotion interface. J Visual Comput Animat. 2003; 14:243–52.

12. Yano H, Tamefusa S, Tanaka N, Saito H, Iwata H. Interactive gait rehabilitation system with a locomotion interface for training patients to climb stairs. Presence. 2012; 21:16–30.

13. Tanaka N, Saitou H, Takao T, Iizuka N, Okuno J, Yano H, et al. Effects of gait rehabilitation with a footpad-type locomotion interface in patients with chronic post-stroke hemiparesis: a pilot study. Clin Rehabil. 2012; 26:686–95.

14. Yano H, Tanaka N, Kamibayashi K, Saitou H, Iwata H. Development of a portable gait rehabilitation system for home-visit rehabilitation. ScientificWorldJournal. 2015; 2015:849831.

15. Schwartz I, Sajin A, Fisher I, Neeb M, Shochina M, Katz-Leurer M, et al. The effectiveness of locomotor therapy using robotic-assisted gait training in subacute stroke patients: a randomized controlled trial. PM R. 2009; 1:516–23.

16. Tanaka N, Ebihara K, Ebata Y, Yano H. Effect of gait rehabilitation with a footpad-type locomotion interface on gait ability in subacute stroke patients. NeuroRehabilitation. 2022; 50:401–7.

17. Molteni F, Gasperini G, Cannaviello G, Guanziroli E. Exoskeleton and end-effector robots for upper and lower limbs rehabilitation: narrative review. PM R. 2018; 10(9 Suppl 2):S174–88.

18. Goffredo M, Iacovelli C, Russo E, Pournajaf S, Di Blasi C, Galafate D, et al. Stroke gait rehabilitation: a comparison of end-effector, overground exoskeleton, and conventional gait training. Appl Sci. 2019; 9:2627.

19. Lee HJ, Lee SH, Seo K, Lee M, Chang WH, Choi BO, et al. Training for walking efficiency with a wearable hip-assist robot in patients with stroke: a pilot randomized controlled trial. Stroke. 2019; 50:3545–52.

20. Mehrholz J, Thomas S, Werner C, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2017; 5:CD006185.
21. Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009; 23:5–13.
22. Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke. 2008; 39:1786–92. Erratum in: Stroke 2008;39:e143.
23. Mayr A, Quirbach E, Picelli A, Kofler M, Smania N, Saltuari L. Early robot-assisted gait retraining in non-ambulatory patients with stroke: a single blind randomized controlled trial. Eur J Phys Rehabil Med. 2018; 54:819–26.
24. Federici S, Meloni F, Bracalenti M, De Filippis ML. The effectiveness of powered, active lower limb exoskeletons in neurorehabilitation: a systematic review. NeuroRehabilitation. 2015; 37:321–40.
25. Hidler JM, Wall AE. Alterations in muscle activation patterns during robotic-assisted walking. Clin Biomech (Bristol, Avon). 2005; 20:184–93.
26. Coenen P, van Werven G, van Nunen MP, Van Dieën JH, Gerrits KH, Janssen TW. Robot-assisted walking vs overground walking in stroke patients: an evaluation of muscle activity. J Rehabil Med. 2012; 44:331–7.

27. Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys Ther. 1984; 64:35–40.
28. Chacko KA. AHA Medical/Scientific Statement: 1994 revisions to classification of functional capacity and objective assessment of patients with diseases of the heart. Circulation. 1995; 92:2003–5.
29. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12:189–98.
30. Iwata H, Yano H, Nakaizumi F. Gait Master: a versatile locomotion interface for uneven virtual terrain. Paper presented at: IEEE Virtual Reality 2001; 2001 Mar 13-17; Yokohama, Japan.
31. Yano H, Tamefusa S, Tanaka N, Saitou H, Iwata H. Gait rehabilitation for stair climbing with a locomotion interface. Paper presented at: 2009 IEEE International Conference on Rehabilitation Robotics; 2009 Jun 23-26; Kyoto, Japan.
32. Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000; 10:361–74.

33. Perry J, Burnfield J. Gait analysis: normal and pathological function. 2nd ed. West Deptford: Slack Incorporated;2010. p. 4–16.
34. Prosser LA, Stanley CJ, Norman TL, Park HS, Damiano DL. Comparison of elliptical training, stationary cycling, treadmill walking and overground walking. Electromyographic patterns. Gait Posture. 2011; 33:244–50.

35. van Kammen K, Boonstra AM, van der Woude LHV, Reinders-Messelink HA, den Otter R. Differences in muscle activity and temporal step parameters between Lokomat guided walking and treadmill walking in post-stroke hemiparetic patients and healthy walkers. J Neuroeng Rehabil. 2017; 14:32.

36. Semaan MB, Wallard L, Ruiz V, Gillet C, Leteneur S, Simoneau-Buessinger E. Is treadmill walking biomechanically comparable to overground walking? A systematic review. Gait Posture. 2022; 92:249–57.

37. Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006; 37:872–6.

38. Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007; 88:43–9.

Table 1.
Demographic characteristics of the RAGT and CGT groups
Table 2.
Changes in muscle activity between pre- and post-training for each group




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download