Abstract
Objective
To examine (1) the location of brain lesion that would predict post-traumatic delirium and (2) the association between volume of brain lesion and occurrence of delirium in patients with traumatic brain injury (TBI).
Methods
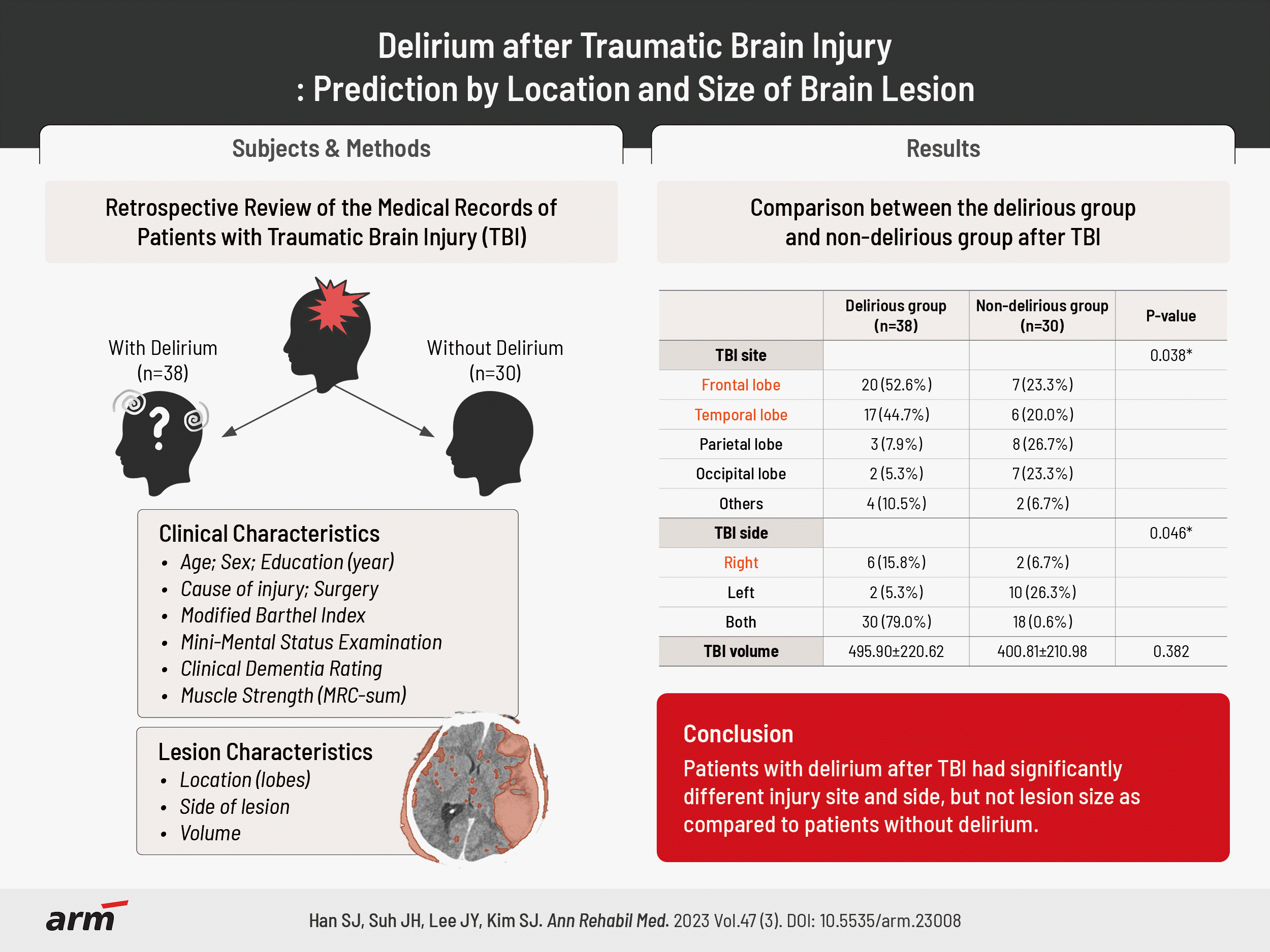
A retrospective study was conducted by reviewing medical records of 68 TBI patients, categorized into two groups: the delirious group (n=38) and non-delirious group (n=30). The location and volume of TBI were investigated with the 3D Slicer software.
Results
The TBI region in the delirious group mainly involved the frontal or temporal lobe (p=0.038). All 36 delirious patients had brain injury on the right side (p=0.046). The volume of hemorrhage in the delirious group was larger by about 95 mL compared to the non-delirious group, but this difference was not statistically significant (p=0.382).
Traumatic brain injury (TBI) is defined as an alteration in brain function and consciousness that results in physical and cognitive impairments caused by an external force [1]. TBI is now perceived as a global public health epidemic and is expected to become the world’s leading cause of neurological disability across all age groups, according to the World Health Organization. It has been reported that on average 1.7 million traumatic brain injuries occur annually in the United States, approximately 275,000 are on admission, 52,000 die, and approximately 76 billion dollars in annual costs arise directly or indirectly from TBI. It can lead to both short- and long-term cognitive functional decline, with a subsequent burden on patients and their relatives.
One of the common early cognitive dysfunction is post-traumatic delirium (PTD). Delirium is an acute disturbance of consciousness and cognition that can occur after a traumatic event such as TBI. Nakase-Thompson et al. [2] revealed delirium in 69.4% of patients with TBI. The cardinal features of delirium include a rapid onset or fluctuating course, inattention, disturbance in the sleep-wake cycle, disorientation, altered level of consciousness, disorganized thoughts with perceptual disturbances, and incoherent speech [3]. Currently, the evaluation of delirium in patients adheres to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) classification, and its diagnostic criteria consist of manifestations such as sleep-wake cycle disturbance, abnormal motor behavior, lability of mood, perceptual disturbance, delusions, and hallucinations [4].
Despite the prevalence of TBI and the significant impact that delirium can have on patient outcomes, the association between the site or severity of brain lesions as a result of trauma and the PTD is not well understood. Delirium in TBI can be derived from several anatomical and functional disruptions, such as altered interregional connectivity among large-scale brain networks for higher cognitive functions, such as attention, emotional integration, and behavioral coherence [4]. Thus, structural brain changes after TBI, which result in cognitive and physical performance following injury, should be considered in the evaluation of PTD. However, there is a lack of research that has systematically explored the relationship between brain lesions and the development and progression of delirium in patients with TBI.
Therefore, this study aimed to determine the correlation between the location and size of brain lesions after TBI and the clinical course of PTD. The following hypotheses were considered: (1) the location of brain lesions would be predictive of diagnosing delirium and (2) the volume of brain lesions would be correlated with the degree of cognitive or physical dysfunction in delirious patients with TBI.
Medical records of patients with TBI admitted to the rehabilitation unit of Ewha Womans University Mokdong Hospital, Seoul, Korea from March 2011 to October 2021 were retrospectively collected. All included patients were older than 18 years and were diagnosed with intracranial hemorrhage after trauma using computerized tomography. The exclusion criteria were the presence of a history of previous stroke or brain injury and a disorder of dementia or mental illness that would confound delirium. We also excluded patients who were not eligible for estimating intellectual ability due to their remaining minimally conscious status. A total of 88 patients with TBI were recruited, and 68 patients who met the criteria were reviewed. The characteristics of these patients, such as age, sex, years of education, trauma type, and brain surgery, were evaluated.
Prior to the start of the study, ethical approval was obtained from a Institutional Review Board (IRB) of Ewha Womans University Mokdong Hospital, Seoul, Korea (IRB No. 2022-03-013) and informed consent was waived due to the retrospective nature of the medical record review. This study was conducted in accordance with the Declaration of Helsinki of the World Medical Association Declaration. It was performed in accordance with the approved guidelines.
In this retrospective study, the presence of delirium in patients with TBI was investigated from transfer to the rehabilitation unit until discharge. An experienced rehabilitation medicine physician evaluated the cases and sorted them into two groups based on the DSM-5 criteria: the delirious group and the non-delirious group. Additionally, in the delirious group, Delirium Rating Scale-Revised-98 (DRS-R-98) was used to assess delirium severity and track its progression, dependent on each TBI patient’s medical records including hospitalization course, nursing chart, application of physical restraint, and history of antipsychotics use. The DRS-R-98 non-cognitive (items 1–8) and cognitive (items 9–13) subscale scores were computed. Eight symptoms of the DRS-R-98, which belong to the non-cognitive domain, were sleep–wake cycle disturbance, perceptual disturbances and hallucinations, delusions, lability of affect, language, thought process abnormalities, motor agitation, and motor retardation. The five symptoms of the DRS-R-98 cognitive domain were orientation, attention, short-term memory, long-term memory, and visuospatial ability. The DRS-R-98 scale consisted of these 16 items, including three diagnostic item scores (e.g., temporal onset, physical disorder and fluctuation of symptom severity), and a maximum total scale score of 46, where each symptom ranged from 0 to 3, normal to severe, or the most disturbed behaviors [5].
The comparison between the delirious and non-delirious groups among patients with TBI was routinely performed using the Mini Mental State Exam (MMSE), Clinical Dementia Rating (CDR), Modified Barthel Index (MBI), to determine the cognitive and Medical Research Council (MRC)-sum score. These measures were used to determine the cognitive and physical functional outcomes of each group from transfer to discharge, based on trauma date. Two physical therapists with more than 5 years of experience evaluated the MMSE, CDR, and MBI. Four physical therapists with more than 5 years of experience evaluated the MRC-sum score. All enrolled patients underwent these examinations, and there was no missing data.
The location of traumatic brain lesions is divided into frontal, temporal, parietal, and occipital lesion derived from the brain’s four basic lobes. And the volume of TBI were demarcated using the 3D Slicer software (Fig. 1). 3D Slicer is a free open-source software platform for biomedical research (http://www.slicer.org). We measured intracranial hemorrhage volume with the 3D Slicer, which could count all pixels compromising the hemorrhage beyond the configuration of the hematoma, in comparison with the traditional planimetric method. Hemorrhage was automatically identified pixel-by-pixel in each slice after setting a threshold range of 50–100 Hounsfield units. In this manner, it went beyond the limits of the size-dependent and shape-dependent estimation errors of the Tada (ABC/2) formula [6,7]. The validity of 3D Slicer has been demonstrated in other studies [8,9].
We performed statistical analysis using IBM SPSS Statistics version 22.0 (IBM Corp., Armonk, NY, USA), using parametric methods. The distribution of continuous outcomes was evaluated using the Shapiro–Wilk test, while the Student’s t-test or Mann–Whitney U-test were employed when necessary. Mean±standard deviation or median (1st quartile–3rd quartile) was reported for parametric and non-parametric data, respectively. The categorical data was expressed as frequency and percentage and the chi-square test was conducted to compare categorical variables. If the number of cells with an expected frequency of less than 5 was over 20%, we used Fisher’s exact test.
In this study, 68 patients with TBI were recruited. These patients were classified into two groups according to the DSM-5 criteria: the delirious group (n=38) and the non-delirious group (n=30). We found a statistically significant difference between the two groups in the TBI site and side (Table 1). Most TBI lesions in the frontal or temporal lobe revealed delirious features (p=0.038). Thirty-seven patients with delirious TBI had frontal or temporal lobe lesion, whereas 13 patients had non-delirious TBI lesions. In addition, all 36 delirious patients after TBI had brain injury on the right side (p=0.046). Six delirious patients had pure right-sided TBI, and 30 delirious patients had TBI on both sides. Meanwhile, in the non-delirious group, only two patients had right-sided TBI, 10 patients had it on left side, and 18 patients had injury on both sides. There were no statistical differences in age, sex, education, trauma type, brain surgical procedure, CDR, MBI, MMSE, and MRC-sum score between the two groups (Table 1).
The TBI volume in delirious group was expected to be larger than that in non-delirious group. The TBI volume was 495.90±220.62 mL in the delirious group and 400.81±210.98 mL in the non-delirious group in brain computed tomography obtained within one day after trauma via 3D Slicer; the hemorrhage volume of the delirious group was larger by about 95 mL than that of the non-delirious group, but the relationship was not statistically significant (p=0.382; Table 1).
As brain hemorrhage resolved, there were statistically significant improvements in DRS-R-98 total, cognitive, and non-cognitive scores in delirious patients with TBI (p=0.014, 0.008, and 0.015, respectively; Table 2). Orientation, attention, and long-term memory were enhanced in the cognitive domain in delirious patients with TBI other than non-delirious patients with TBI from transfer until discharge, based on injury date (Table 2).
The medications used for delirium in the delirious patients with TBI included quetiapine (n=29), trazodone (n=8), risperidone (n=7), paroxetine (n=6), triazolam (n=4), lorazepam (n=5), peridol (n=2), alprazolam (n=2), and benzatropine (n=2). The most commonly used drug was quetiapine, with an average daily dose of 25.34 mg, and the second most commonly used drug was trazodone, with an average daily dose of 15.13 mg. On average, these two drugs were used for 45 days. The use of these drugs might have affected the clinical course of delirium in patients with TBI. There were no adverse or side effects reported during the administration period of antipsychotics in these patients.
Understanding the pathophysiology of delirium in TBI is crucial for improving patient outcomes and reducing the burden of TBI on individuals and society as a whole. We found that damage to the frontal or temporal lobe was frequently found in delirious patients with TBI. The frontal lobe and limbic system are closely linked structures that are involved in attention, emotional regulation, and stress response. Limbic structures, such as the hippocampus, modulate attention and conscious awareness, which helps organisms maintain arousal [10,11]. The temporal lobe including hippocampus and parahippocampal gyrus have been implicated in awareness specifically with respect to operations supporting declarative memory [12]. Abnormalities in these areas can paint the clinical picture of a delirious patient: disturbance in attention and awareness with changed cognition or the development of perceptual disturbance [12,13]. The location of the brain injury could have a significant prognostic value in predicting delirium. Indeed, the delirious patients with TBI in this study had damage in orientation and attention, which improved as delirium resolves, as shown in the results of DRS-R-98.
Furthermore, delirious features were observed in patients with TBI, particularly in those with lesions in the right hemisphere. Previous studies have analyzed the odds ratio of delirium in patients with brain lesions, but these studies were mainly limited to patients with stroke rather than trauma. While there were more stroke patients who did not develop delirium among all stroke patients, patients with TBI had a higher rate of delirium, with more than half of them developing delirium in other studies [14]. This study is noteworthy because it specifically focused on patients with TBI, where delirium is common. According to our study outcomes, the presence of delirium in TBI is not related to age, sex, education, trauma type, brain surgery, or size of brain injury, but rather to the location and side of the brain injury. These clinical characteristics, including TBI volume, have not offered a plausible explanation for delirium in patients with traumatic brain hemorrhage, whereas the site of hemorrhage may provide an attractive explanation.
In TBI, focal hemorrhagic contusions have preference sights in the frontal and temporal lobes [15]. A study by van der Naalt et al. [16] suggests that twice as many lesions were seen in patients with restlessness and agitation, mainly localized in the frontotemporal region after head injury. These previous studies investigated the delirious features of patients with TBI and the location of the TBI site, but they did not measure the TBI volume or differentiate the TBI side, unlike our study. Moreover, the delirious features were described using the Glasgow Coma Scale, which is a traditional tool to measure consciousness, rather than a screening tool for delirium. In contrast, our study examined the presence of delirium by using the DSM-5 criteria and evaluated the severity of delirium in patients with TBI in detail, using the DRS-R-98.
The susceptibility of certain anatomical regions to stroke or of certain types of neurological deficits to delirium has been reported in several studies and case reports. Previous studies have revealed that post-stroke delirium may correlate with issues in the right hemisphere, right frontal straight gyrus, or medial occipitotemporal lobe [14]. Specific brain structures involved in delirium are elucidated, like the prefrontal cortex, thalamus, and basal ganglia, especially in the non-dominant hemisphere [17]. Oldenbeuving et al. [18] suggested that post-stroke delirium is more frequent in patients with right hemispheric stroke than in those with left. Our study concluded that right-hemispheric TBI has a stronger association with delirium. Attention, the impairment of which is an important feature of delirium, is predominantly right hemisphere-dependent [19]. Identifying TBI lesion site and side could contribute to predicting a higher risk for delirium.
Existing studies have addressed delirium by measuring the volume of the remaining brain volume or intracerebral hematoma (ICH) volume. The ABC/2 formula has been widely used for volume assessment of ICH; nonetheless, it cannot analyze other types of intracranial hemorrhages, such as epidural hemorrhage, subdural hemorrhage, and subarachnoid hemorrhage, which are linked to the possibility of delirium in TBI. Furthermore, the ABC/2 formula is crude for irregularly shaped hematomas, and hematomas are often irregular rather than ellipsoid. Its accuracy decreases with large, irregular or lobar hematomas that are more likely to occur in traumatic conditions [7].
Accurate measurement of hemorrhage volume is a chief concern since hematoma volume alters treatment strategy, functional outcome, and mortality. In our study, various hemorrhage types and volumes were included and calculated using radiologic 3D Slicer, which is different from other studies.
Other studies have reported no significant difference between hematoma volumes in ever-delirious and never-delirious patients [11,20]. A study reported that delirious patients had an ICH volume approximately twice that of non-delirious patients [21]. However, the above-mentioned studies merely dealt with ICH and did not consider other types of intracranial hematomas. Our study suggests that the degree of hematoma volume, including all types of cerebral hemorrhage, is independent of delirious symptoms.
If delirium is present, it is difficult to proceed with rehabilitation treatment and TBI patients with delirium are often excluded from rehabilitation treatment. In this study, orientation, attention, and long-term memory were enhanced in the cognitive domain in delirious patients with TBI other than non-delirious patients with TBI. All patients included in this study underwent rehabilitation treatment. In previous study, delirium and physical function are closely related, since disability, immobility and declined function are identified as risk factors of delirium [22]. In this study, the improvement in cognition of TBI patients with delirium could be the effect of active rehabilitation treatment. Therefore, it would be helpful to include TBI patients with delirium in active rehabilitation to improve prognosis.
We have several limitations in this study. First, our study results cannot be generalized to all patients with TBI because the study population was derived from a single ward that met the selection criteria. Second, there were variations in the risk factors for delirium such as alcohol use, comorbidities, hospital length of stay, that were not included. Therefore, further study with a larger sample size or a longer period of survey is recommended to understand the pathophysiology of PTD. Due to its retrospective manner, this study lacked various evaluation tools.
In conclusion, patients with TBI developed delirium, most of whom had frontal or temporal lobe lesion and right-sided brain injury. Compared with non-delirious group, the delirious group after TBI had significantly different injury site and side without a difference in lesion size. The findings of this study have the potential to improve understanding of the pathophysiology of delirium in TBI. In this study, orientation, attention, and long-term memory were enhanced in the cognitive domain in delirious patients with TBI other than non-delirious patients with TBI. Therefore, it would be helpful to include patients with PTD in active rehabilitation to improve prognosis.
Notes
Conceptualization: Han SJ, Suh JH, Kim SJ, Lee JY. Methodology: Han SJ, Suh JH, Kim SJ, Lee JY. Formal analysis: Han SJ, Suh JH, Kim SJ. Project administration: Han SJ. Visualization: Kim SJ. Writing – original draft: Kim SJ. Writing – review and editing: Han SJ, Lee JY, Suh JH. Approval of final manuscript: all authors.
REFERENCES
1. Menon DK, Schwab K, Wright DW, Maas AI; Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010; 91:1637–40.

2. Nakase-Thompson R, Sherer M, Yablon SA, Nick TG, Trzepacz PT. Acute confusion following traumatic brain injury. Brain Inj. 2004; 18:131–42.

3. Kluger C, Shah P, Maiti S, Babalola O, Mulvany C, Sinvani L. Therapeutic advances in the prevention and treatment of delirium in the hospital setting. Am J Ther. 2018; 25:e3–14.

4. Ganau M, Lavinio A, Prisco L. Delirium and agitation in traumatic brain injury patients: an update on pathological hypotheses and treatment options. Minerva Anestesiol. 2018; 84:632–40.

5. Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci 2001;13:229-42. Erratum in: J Neuropsychiatry Clin Neurosci. 2001; 13:433.
6. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 27:1304–5.

7. Webb AJ, Ullman NL, Morgan TC, Muschelli J, Kornbluth J, Awad IA, et al. Accuracy of the ABC/2 score for intracerebral hemorrhage: systematic review and analysis of MISTIE, CLEAR-IVH, and CLEAR III. Stroke. 2015; 46:2470–6.
8. Xu X, Chen X, Zhang J, Zheng Y, Sun G, Yu X, et al. Comparison of the Tada formula with software slicer: precise and low-cost method for volume assessment of intracerebral hematoma. Stroke. 2014; 45:3433–5.

9. Chen M, Li Z, Ding J, Lu X, Cheng Y, Lin J. Comparison of common methods for precision volume measurement of hematoma. Comput Math Methods Med. 2020; 2020:6930836.

10. Larsen LK, Møller K, Petersen M, Egerod I. Delirium prevalence and prevention in patients with acute brain injury: a prospective before-and-after intervention study. Intensive Crit Care Nurs. 2020; 59:102816.

11. Naidech AM, Polnaszek KL, Berman MD, Voss JL. Hematoma locations predicting delirium symptoms after intracerebral hemorrhage. Neurocrit Care. 2016; 24:397–403.

12. Boukrina O, Barrett AM. Disruption of the ascending arousal system and cortical attention networks in post-stroke delirium and spatial neglect. Neurosci Biobehav Rev. 2017; 83:1–10.

13. Kalvas LB, Monroe TB. Structural brain changes in delirium: an integrative review. Biol Res Nurs. 2019; 21:355–65.

14. Qu J, Chen Y, Luo G, Zhong H, Xiao W, Yin H. Delirium in the acute phase of ischemic stroke: incidence, risk factors, and effects on functional outcome. J Stroke Cerebrovasc Dis. 2018; 27:2641–7.

15. Pavlovic D, Pekic S, Stojanovic M, Popovic V. Traumatic brain injury: neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary. 2019; 22:270–82.

16. van der Naalt J, van Zomeren AH, Sluiter WJ, Minderhoud JM. Acute behavioural disturbances related to imaging studies and outcome in mild-to-moderate head injury. Brain Inj. 2000; 14:781–8.

17. Veiga Fernández F, Cruz Jentoft AJ. [Delirium: etiology and pathophysiology]. Rev Esp Geriatr Gerontol 2008;43 Suppl 3:4-12. Spanish. Erratum in: Rev Esp Geriatr Gerontol. 2009; 44:56.
18. Oldenbeuving AW, de Kort PL, Jansen BP, Algra A, Kappelle LJ, Roks G. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology. 2011; 76:993–9.

19. Mizuno M. Neuropsychological characteristics of right hemisphere damage: investigation by attention tests, concept formation and change test, and self-evaluation task. Keio J Med. 1991; 40:221–34.

20. Naidech AM, Beaumont JL, Rosenberg NF, Maas MB, Kosteva AR, Ault ML, et al. Intracerebral hemorrhage and delirium symptoms. Length of stay, function, and quality of life in a 114-patient cohort. Am J Respir Crit Care Med. 2013; 188:1331–7.

21. Reznik ME, Drake J, Margolis SA, Moody S, Murray K, Costa S, et al. Deconstructing poststroke delirium in a prospective cohort of patients with intracerebral hemorrhage. Crit Care Med. 2020; 48:111–8.

22. Laurila JV, Laakkonen ML, Tilvis RS, Pitkala KH. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res 2008;65:249-54. Erratum in: J Psychosom Res. 2008; 65(5):507.
Fig. 1.
(A) Brain hemorrhage after trauma shown in computerized tomography. (B) Traumatic brain injury volume assessment with 3D Slicer.
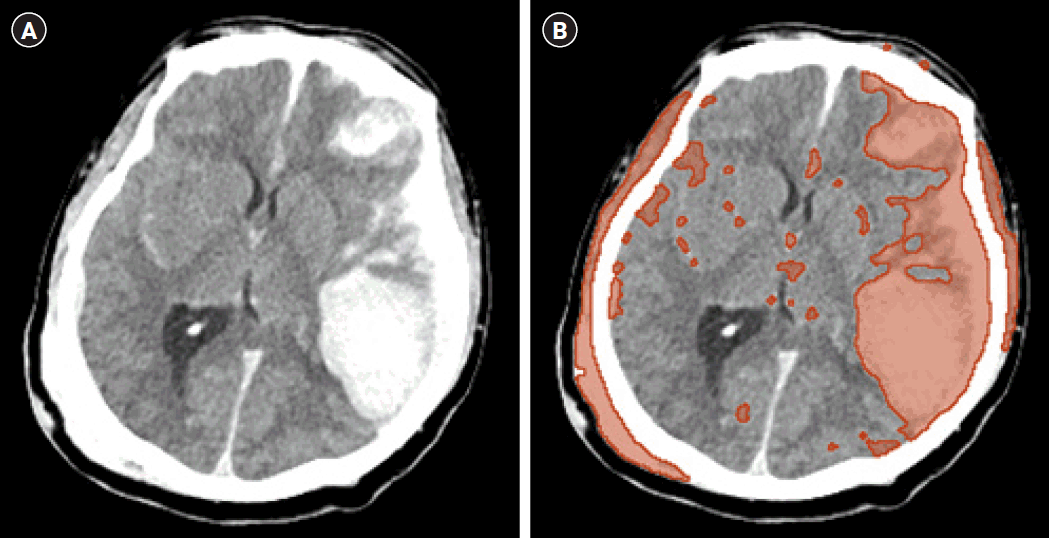
Table 1.
Comparison between the characteristics of delirious group and non-delirious group after TBI
| Delirious group (n=38) | Non-delirious group (n=30) | p-value | |
|---|---|---|---|
| Age (yr) | 65.84±10.98 | 66.82±13.48 | 0.688 |
| Age group | 0.516 | ||
| <65 yr | 12 (31.6) | 10 (33.3) | |
| ≥65 yr | 26 (68.4) | 20 (66.7) | |
| Sex | 0.063 | ||
| Male | 29 (76.3) | 17 (56.7) | |
| Female | 9 (23.7) | 13 (43.3) | |
| Education (yr) | 10.41±4.38 | 10.67±3.74 | 0.878 |
| Trauma type | 0.773 | ||
| Traffic accident | 14 (36.8) | 12 (40.0) | |
| Fall | 17 (44.7) | 12 (40.0) | |
| Unknown | 7 (18.5) | 6 (20.0) | |
| Surgical procedure | 0.383 | ||
| Brain surgery | 24 (63.2) | 17 (56.7) | |
| No brain surgery | 14 (36.8) | 13 (43.3) | |
| TBI site | 0.038* | ||
| Frontal lobe | 20 (52.6) | 7 (23.3) | |
| Temporal | 17 (44.7) | 6 (20.0) | |
| Parietal | 3 (7.9) | 8 (26.7) | |
| Occipital | 2 (5.3) | 7 (23.3) | |
| Others | 4 (10.5) | 2 (6.7) | |
| TBI side | 0.046* | ||
| Right | 6 (15.8) | 2 (6.7) | |
| Left | 2 (5.3) | 10 (33.3) | |
| Both | 30 (78.9) | 18 (60.0) | |
| TBI volume (mL) | 495.90±220.62 | 400.81±210.98 | 0.382 |
| Clinical Dementia Rating | 2 (1–2) | 1 (1–3) | 0.421 |
| MBI score | 38.66±22.40 | 40.44±28.69 | 0.779 |
| MMSE score | 13.04±8.14 | 15.05±8.48 | 0.248 |
| MRC-sum score | 46.01±7.13 | 42.14±10.88 | 0.065 |
Table 2.
Comparison between the initial and follow-up DRS-R-98 scores in the delirious group after traumatic brain injury
| Delirious group (n=38) | p-value | |||
|---|---|---|---|---|
| Initial score | Follow-up score | △Score | ||
| DRS-R-98 | ||||
| Total (n=38) | 20.67±8.43 | 13.55±2.85 | 5.10 | 0.014* |
| Cognitive | 13.68±5.51 | 6.33±4.22 | 3.44 | 0.008* |
| Non-cognitive | 4.88±2.75 | 4.83±0.82 | 0.31 | 0.015* |
| Cognitive domain | ||||
| Orientation | 2.13±0.74 | 1.23±0.84 | 1.03 | 0.014* |
| Attention | 2.51±0.78 | 1.60±0.76 | 1.04 | 0.010* |
| Short-term memory | 0 (0–0) | 0 (0–0) | 0.07 | 0.322 |
| Long-term memory | 2.43±0.52 | 1.72±1.60 | 0.71 | 0.011* |
| Visuospatial ability | 2.10±1.39 | 1.83±1.02 | 0.68 | 0.482 |
| Non-cognitive domain | ||||
| Sleep–wake cycle disturbance | 2.35±1.14 | 0.98±0.68 | 0.14 | 0.009* |
| Perceptual disturbances | 0 (0–0) | 0 (0–0) | 0.11 | 0.284 |
| Delusions | 1 (1–2) | 0 (0–1) | 1.01 | 0.118 |
| Lability of affect | 1 (1–2) | 0 (0–2) | 0.73 | 0.192 |
| Thought process abnormalities | 0 (0–0) | 0 (0–0) | 0.01 | 0.543 |
| Motor agitation | 2 (1–3) | 1 (1–2) | 0.50 | 0.098 |
| Motor retardation | 0.38±0.88 | 0.25±0.41 | 0.10 | 0.453 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download