This article has been
cited by other articles in ScienceCentral.
Abstract
The World Health Organization (WHO) updated the classification of pituitary tumors in 2022. The new classification presents detailed histological subtyping of a pituitary neuroendocrine tumor (PitNET) based on the tumor cell lineage, cell type, and related characteristics. The immunohistochemistry for pituitary transcription factors (PIT1, TPIT, SF1, GATA3, and ERα) is routinely needed in this classification. The controversy regarding the change of behavior code of all PitNET/pituitary adenoma from “0” for benign tumors to “3” for primary malignant tumors is a topic of debate among experts, nowadays. Some authors represent that pituitary adenoma has a tendency for hemorrhage and necrosis and frequent invasion of the cavernous sinus. However, most small PitNET/pituitary adenoma do not need any treatment because of benign biologic behavior or less than 5% recurrence after gross total removal. Pituitary apoplexy is also benign nature but has a tendency of cranial nerve compression or panhypopituitarism. Most of cavernous invasion is compression of the cavernous sinus. Aggressive PitNET/pituitary adenoma with malignant biological behavior is less than 1%.
Keywords: Pituitary gland, Neuroendocrine tumor, Pituitary adenoma
INTRODUCTION
The 5th edition of the World Health Organization (WHO) classifications—2021 World Health Organization Classification of Central Nervous System Tumors and 2022 World Health Organization Classification of Endocrine and Neuroendocrine Tumors—has made significant changes to the classification of pituitary adenomas, the most common type of pituitary gland tumor as determined by expression of transcription factors, hormones, and other biomarkers [
12]. In this paper, we will discuss the clinical, histopathological, and radiological disease character.
DEFINITION AND NOMENCLATURE CHANGE
Pituitary neuroendocrine tumor (PitNET)/pituitary adenoma is defined as a clonal neoplastic proliferation of the anterior pituitary hormone-producing cells [
2], and the third most common tumor after meningiomas and diffuse glial tumors [
3]. A major nomenclature change from the previous edition of the WHO classification is the transition from “pituitary adenoma” to “pituitary neuroendocrine tumor” (PitNET) [
1]. Adenomas are benign, and it means a mass of cells (tumor) that does not invade neighboring tissue or metastasize (spread throughout the body). Compared to malignant (cancerous) tumors, benign tumors generally have a slower growth rate and relatively well-differentiated cells.
NEW (5TH EDITION) WHO CLASSIFICATIONS
A classification of the pituitary tumor has been changed to neuroendocrine tumors, rather than organ-specific classification [
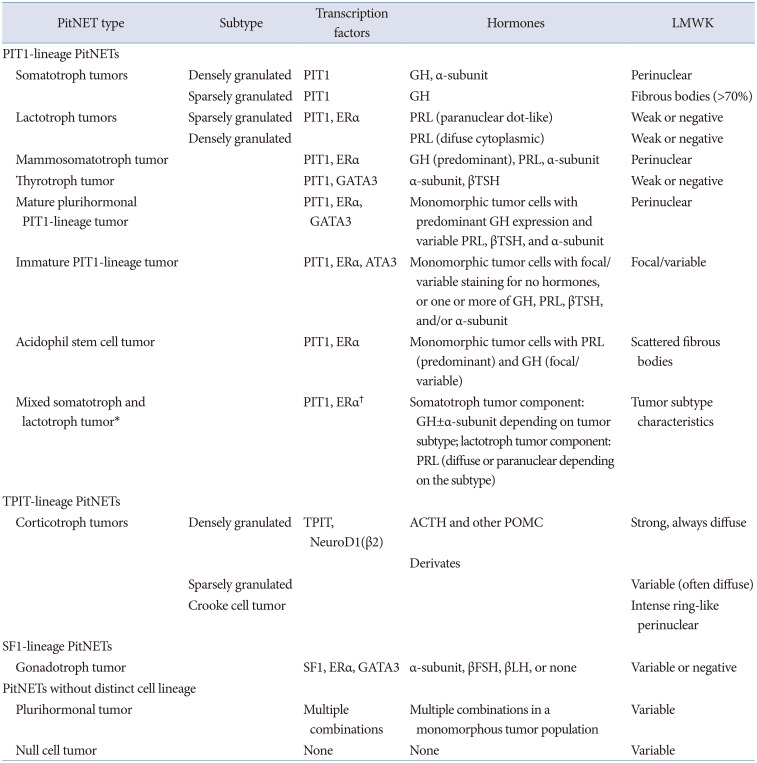
4]. In the WHO Classification of Central Nervous System Tumors, 5th edition released in 2021, “pituitary adenoma” was incorporated under the same entry as “PitNET.” In the WHO Classification of Endocrine and Neuroendocrine Tumors 5th edition released in 2022, it is listed as “PitNET/pituitary adenoma.” The adenohypophysis is composed of at least six normal cell types: somatotrophs, lactotrophs, mammosomatotrophs, and thyrotrophs are of PIT1 lineage, corticotrophs are of TPIT lineage, and gonadotrophs are of SF1 lineage (
Table 1) [
15]. The pathological diagnosis should include pituitary neuroendocrine tumor, transcription factor (PIT1, TPIP, SF1), and the name of the cell from which it differentiated, such as “A well-differentiated pituitary neuroendocrine tumor composed of PIT1-lineage adenohypophysial cells with mammosomatotroph differentiation.”
In the 5th edition, the behavior code for International Classification of Diseases for Oncology (ICD-O) was revised from “0” for benign tumors to “3” for primary malignant tumors as same to neuroendocrine tumors in other organs [
12]. In the 3rd edition of ICD-O released in 2001, morphology code of pituitary adenoma was M8272/0 and pituitary carcinoma was M8272/3. In ICD-11 code released on January 2023, pituitary neuroendocrine tumor is classified into three different codes: 1) 2F37.Z benign neoplasm of endocrine glands, unspecified (pituitary gland); 2) 2F7A.Z neoplasm of uncertain behavior of endocrine glands, unspecified (pituitary gland); and 3) 2D12Z malignant neoplasm of other endocrine glands or related structures, unspecified (pituitary gland).
CONTROVERSY OF PitNET/PITUITARY ADENOMA
The WHO classification of pituitary tumors was updated in 2022. The new classification provides detailed histological subtyping of a PitNET based on the tumor cell lineage, cell type, and related characteristics [
5]. The immunohistochemistry for pituitary transcription factors (PIT1, TPIT, SF1, GATA3, and ERα) is routinely needed in this classification.
The controversy regarding the change of behavior code of all PitNET/pituitary adenoma from “0” for benign tumors to “3” for primary malignant tumors is a topic of debate among experts, nowadays [
6]. We neurosurgeons all know that there are few patients with multiple recurrences with malignant tendency or aggressive nature. Some authors noted that pituitary adenoma has a tendency for hemorrhage and necrosis, frequent invasion of cavernous sinus, and rare metastasis [
7]. However, the biology of pituitary adenoma is clinically benign. An average prevalence of pituitary adenoma was reported to be 10% in a comprehensive review of 16 studies, more than 21,000 autopsies [
8]. There are several studies that 10%–40% of normal volunteers have discrete pituitary lesions with brain MRI scan [
910]. It means overall population prevalence of pituitary adenoma is about more than 10%. Recurrence rates were approximately 20%–50% after surgical resection of nonfunctioning pituitary adenoma over 10 years of follow-up periods but no mortality or overall survival was discussed because of benign biology of pituitary adenoma [
11]. Most small PitNET/pituitary adenoma do not need any treatment because of benign biologic behavior or less than 5% recurrence after gross total removal. Pituitary apoplexy is also benign nature but has a tendency of cranial nerve compression or panhypopituitarism. Most of cavernous invasion is compression of the cavernous sinus. Melmed et al. [
12] reported that fewer than one-thousandth of all pituitary adenomas cause clinically significant disease and cellular senescence acts as a mechanistic buffer protecting against malignant transformation, an extremely rare event. As a personal experience, I had only two (<1%) malignant progression cases over two hundred pituitary tumors in twenty years periods and no metastasis. Aggressive PitNET/pituitary adenoma with malignant biological behavior is less than 1%. Meningiomas also have a frequent invasion to cavernous sinus and venous sinus but behavior code of benign meningioma is M9530/0. Cranial schwannoma has a tendency for hemorrhage and invasion of cavernous sinus, but behavior code is M9560/0. So pituitary adenoma is more suitable for defining this disease since it originated from the anterior lobe of the pituitary gland and a large majority of the tumors are well-differentiated and benign neoplasms that do not adversely impact life expectancy [
1314].
Patients who have incidentally found tumor without symptoms might have a great psychological burden for the naming of malignant tumor. That malignant behavior code will occur many social, medical, educational, and legal problems especially with the insurance company in medical fields. And this classification system provides no additional treatment policy for neurosurgeons in clinical practice, especially in determining the treatment strategies, such as deciding the follow-up plans and adjunctive treatment.
CONCLUSIONS
The PitNET/pituitary adenoma is the most common pathology of the sellar lesion. The new classification from pituitary adenoma to PitNET based on the tumor cell lineage, cell type, and related characteristics is adjustable. The clinical biology of pituitary adenomas is particularly benign nature and very different from other tumors of the endocrine system, such as thyroid and other neuroendocrine tumors. So, the change of behavior code of all PitNET/pituitary adenoma from “0” for benign tumors to “3” for primary malignant tumors seems debatable. That malignant behavior code will occur many social, medical, educational, and legal fields.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during this study.
References
2. WHO Classification of Tumours Editorial Board. Endocrine and neuroendocrine tumours: WHO classification of tumours series. Volume 10 [Internet]. 5th ed. Lyon: International Agency for Research on Cancer;2022. Accessed Nov 1, 2022. at
https://tumourclassification.iarc.who.int/.
3. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021; 23(12 Suppl 2):iii1–iii105. PMID:
34608945.
4. Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018; 31:1770–1786. PMID:
30140036.
5. Asa SL, Mete O, Perry A, Osamura RY. Overview of the 2022 WHO classification of pituitary tumors. Endocr Pathol. 2022; 33:6–26. PMID:
35291028.
6. Wan XY, Chen J, Wang JW, Liu YC, Shu K, Lei T. Overview of the 2022 WHO classification of pituitary adenomas/pituitary neuroendocrine tumors: clinical practices, controversies, and perspectives. Curr Med Sci. 2022; 42:1111–1118. PMID:
36544040.
7. Tsukamoto T, Miki Y. Imaging of pituitary tumors: an update with the 5th WHO classifications—part 1. Pituitary neuroendocrine tumor (PitNET)/pituitary adenoma. Jpn J Radiol. 2023; 02. 24. [Epub]. Available at: . DOI:
10.1007/s11604-023-01400-7.
8. Molitch ME. Nonfunctioning pituitary tumors. Handb Clin Neurol. 2014; 124:167–184. PMID:
25248587.
9. Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med. 1994; 120:817–820. PMID:
8154641.
10. Chong BW, Kucharczyk W, Singer W, George S. Pituitary gland MR: a comparative study of healthy volunteers and patients with microadenomas. AJNR Am J Neuroradiol. 1994; 15:675–679. PMID:
8010269.
11. Zhang X, Yang F, Han N. Recurrence rate and exploration of clinical factors after pituitary adenoma surgery: a systematic review and meta-analysis based on computer artificial intelligence system. Comput Intell Neurosci. 2022; 2022:6002672. PMID:
36275975.
12. Melmed S, Kaiser UB, Lopes MB, Bertherat J, Syro LV, Raverot G, et al. Clinical biology of the pituitary adenoma. Endocr Rev. 2022; 43:1003–1037. PMID:
35395078.
13. Ho KKY, Fleseriu M, Wass J, van der Lely A, Barkan A, Giustina A, et al. A tale of pituitary adenomas: to NET or not to NET. Pituitary. 2019; 22:569–573. PMID:
31571098.
14. Ho K, Fleseriu M, Kaiser U, Salvatori R, Brue T, Lopes MB, et al. Pituitary neoplasm nomenclature workshop: does adenoma stand the test of time? J Endocr Soc. 2021; 5:bvaa205. PMID:
33604494.
Table 1
The 2022 WHO classification of PitNETs, 5th edition
|
PitNET type |
Subtype |
Transcription factors |
Hormones |
LMWK |
|
PIT1-lineage PitNETs |
|
Somatotroph tumors |
Densely granulated |
PIT1 |
GH, α-subunit |
Perinuclear |
|
Sparsely granulated |
PIT1 |
GH |
Fibrous bodies (>70%) |
|
Lactotroph tumors |
Sparsely granulated |
PIT1, ERα |
PRL (paranuclear dot-like) |
Weak or negative |
|
Densely granulated |
PRL (difuse cytoplasmic) |
Weak or negative |
|
Mammosomatotroph tumor |
|
PIT1, ERα |
GH (predominant), PRL, α-subunit |
Perinuclear |
|
Thyrotroph tumor |
|
PIT1, GATA3 |
α-subunit, βTSH |
Weak or negative |
|
Mature plurihormonal PIT1-lineage tumor |
|
PIT1, ERα, GATA3 |
Monomorphic tumor cells with predominant GH expression and variable PRL, βTSH, and α-subunit |
Perinuclear |
|
Immature PIT1-lineage tumor |
|
PIT1, ERα, ATA3 |
Monomorphic tumor cells with focal/variable staining for no hormones, or one or more of GH, PRL, βTSH, and/or α-subunit |
Focal/variable |
|
Acidophil stem cell tumor |
|
PIT1, ERα |
Monomorphic tumor cells with PRL (predominant) and GH (focal/variable) |
Scattered fibrous bodies |
|
Mixed somatotroph and lactotroph tumor*
|
|
PIT1, ERα†
|
Somatotroph tumor component: GH±α-subunit depending on tumor subtype; lactotroph tumor component: PRL (diffuse or paranuclear depending on the subtype) |
Tumor subtype characteristic |
|
TPIT-lineage PitNETs |
|
Corticotroph tumors |
Densely granulated |
TPIT, NeuroD1(β2) |
ACTH and other POMC |
Strong, always diffuse |
|
Derivates |
|
Sparsely granulated |
|
|
Variable (often diffuse) |
|
Crooke cell tumor |
|
|
Intense ring-like perinuclear |
|
SF1-lineage PitNETs |
|
Gonadotroph tumor |
|
SF1, ERα, GATA3 |
α-subunit, βFSH, βLH, or none |
Variable or negative |
|
PitNETs without distinct cell lineage |
|
Plurihormonal tumor |
|
Multiple combinations |
Multiple combinations in a monomorphous tumor population |
Variable |
|
Null cell tumor |
|
None |
None |
Variable |
Pituitary adenomas/PitNET arising in adenohypophysis express neuroendocrine proteins such as synaptophysin, chromogranin A, and CD56. Pituitary adenomas may be locally invasive and can metastasize. However, there are no morphologic features that distinguish locally invasive and metastatic lesions from benign tumor.
WHO unifies the pituitary adenomas to neuroendocrine tumors (NET) and change the nomenclature. This change in nomenclature is accompanied by a change in behavior coding that aligns with other NET such as gastrointerstinal tract.
The revolution of NET nomenclature and behavior code is proceeding according to WHO policy. All kinds of NET in the whole body have malignant potential, categorized as behavior code/3.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download