1. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996. PMID:
15758009.
2. Roh TH, Park HH, Kang SG, Moon JH, Kim EH, Hong CK, et al. Long-term outcomes of concomitant chemoradiotherapy with temozolomide for newly diagnosed glioblastoma patients: a single-center analysis. Medicine (Baltimore). 2017; 96:e7422. PMID:
28682902.
3. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011; 115:3–8. PMID:
21417701.
4. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001; 95:190–198.
5. Baldock AL, Rockne RC, Boone AD, Neal ML, Hawkins-Daarud A, Corwin DM, et al. From patient-specific mathematical neuro-oncology to precision medicine. Front Oncol. 2013; 3:62. PMID:
23565501.
6. Zetterling M, Roodakker KR, Berntsson SG, Edqvist PH, Latini F, Landtblom AM, et al. Extension of diffuse low-grade gliomas beyond radiological borders as shown by the coregistration of histopathological and magnetic resonance imaging data. J Neurosurg. 2016; 125:1155–1166. PMID:
26918468.
7. Yamahara T, Numa Y, Oishi T, Kawaguchi T, Seno T, Asai A, et al. Morphological and flow cytometric analysis of cell infiltration in glioblastoma: a comparison of autopsy brain and neuroimaging. Brain Tumor Pathol. 2010; 27:81–87. PMID:
21046309.
8. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–466. PMID:
19269895.
9. Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within “noneloquent” areas in the left dominant hemisphere: toward a “supratotal” resection. J Neurosurg. 2011; 115:232–239. PMID:
21548750.
10. Eyüpoglu IY, Hore N, Merkel A, Buslei R, Buchfelder M, Savaskan N. Supra-complete surgery via dual intraoperative visualization approach (DiVA) prolongs patient survival in glioblastoma. Oncotarget. 2016; 7:25755–25768. PMID:
27036027.
11. Di L, Shah AH, Mahavadi A, Eichberg DG, Reddy R, Sanjurjo AD, et al. Radical supramaximal resection for newly diagnosed left-sided eloquent glioblastoma: safety and improved survival over gross-total resection. J Neurosurg. 2022; 138:62–69. PMID:
35623362.
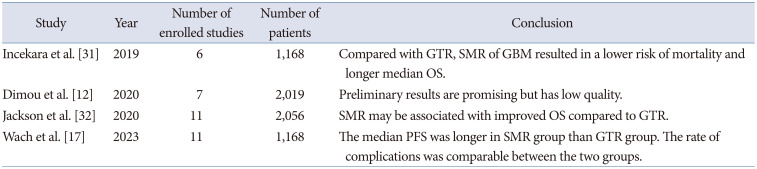
12. Dimou J, Beland B, Kelly J. Supramaximal resection: a systematic review of its safety, efficacy and feasibility in glioblastoma. J Clin Neurosci. 2020; 72:328–334. PMID:
31864830.
13. Glenn CA, Baker CM, Conner AK, Burks JD, Bonney PA, Briggs RG, et al. An examination of the role of supramaximal resection of temporal lobe glioblastoma multiforme. World Neurosurg. 2018; 114:e747–e755. PMID:
29555603.
14. Livermore LJ, Williams S, Clifton L, McCulloch P, Ansorge O, Voets N, et al. Functionally guided supramaximal resection of IDH-wildtype glioblastomas and the effect on progression free survival. Neuro Oncol. 2018; 20(Suppl 5):V346–V347.
15. Certo F, Altieri R, Maione M, Schonauer C, Sortino G, Fiumanò G, et al. FLAIRectomy in supramarginal resection of glioblastoma correlates with clinical outcome and survival analysis: a prospective, single institution, case series. Oper Neurosurg (Hagerstown). 2021; 20:151–163. PMID:
33035343.
16. Tripathi S, Vivas-Buitrago T, Domingo RA, Biase G, Brown D, Akinduro OO, et al. IDH-wild-type glioblastoma cell density and infiltration distribution influence on supramarginal resection and its impact on overall survival: a mathematical model. J Neurosurg. 2021; 136:1567–1575. PMID:
34715662.
17. Wach J, Vychopen M, Kühnapfel A, Seidel C, Güresir E. A systematic review and meta-analysis of supramarginal resection versus gross total resection in glioblastoma: can we enhance progression-free survival time and preserve postoperative safety? Cancers (Basel). 2023; 15:1772. PMID:
36980659.
18. Wang LM, Banu MA, Canoll P, Bruce JN. Rationale and clinical implications of fluorescein-guided supramarginal resection in newly diagnosed high-grade glioma. Front Oncol. 2021; 11:666734. PMID:
34123831.
19. Esquenazi Y, Friedman E, Liu Z, Zhu JJ, Hsu S, Tandon N. The survival advantage of “supratotal” resection of glioblastoma using selective cortical mapping and the subpial technique. Neurosurgery. 2017; 81:275–288. PMID:
28368547.
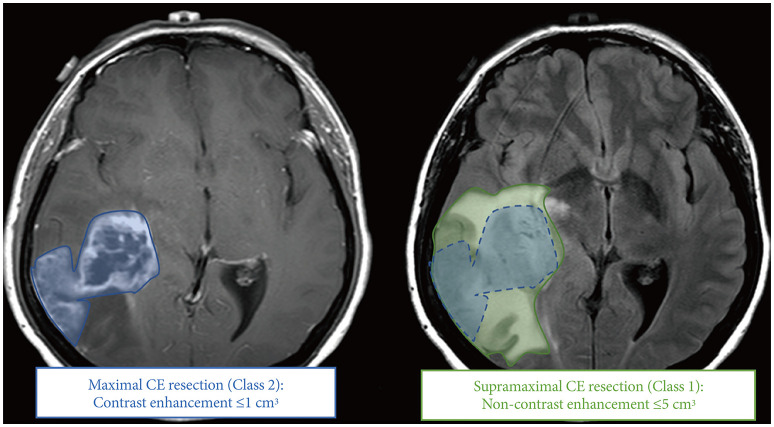
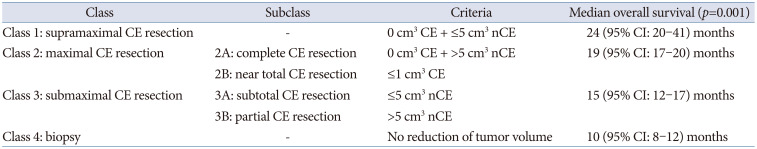
20. Karschnia P, Young JS, Dono A, Häni L, Sciortino T, Bruno F, et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: a report of the RANO resect group. Neuro Oncol. 2023; 25:940–954. PMID:
35961053.
21. Dandy WE. Removal of right cerebral hemisphere for certain tumors with hemiplegia: preliminary report. JAMA. 1928; 90:823–825.
22. Jusue-Torres I, Prabhu VC, Jones GA. Dandy’s hemispherectomies: historical vignette. J Neurosurg. 2021; 135:1836–1842. PMID:
33990086.
23. Wijnenga MMJ, French PJ, Dubbink HJ, Dinjens WNM, Atmodimedjo PN, Kros JM, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol. 2018; 20:103–112. PMID:
29016833.
24. Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014; 16:81–91. PMID:
24305719.
25. Choi J, Kim SH, Ahn SS, Choi HJ, Yoon HI, Cho JH, et al. Extent of resection and molecular pathologic subtype are potent prognostic factors of adult WHO grade II glioma. Sci Rep. 2020; 10:2086. PMID:
32034238.
26. Wang Y, Wang K, Wang J, Li S, Ma J, Dai J, et al. Identifying the association between contrast enhancement pattern, surgical resection, and prognosis in anaplastic glioma patients. Neuroradiology. 2016; 58:367–374. PMID:
26795126.
27. Fujii Y, Muragaki Y, Maruyama T, Nitta M, Saito T, Ikuta S, et al. Threshold of the extent of resection for WHO grade III gliomas: retrospective volumetric analysis of 122 cases using intraoperative MRI. J Neurosurg. 2018; 129:1–9.
28. Hong JB, Roh TH, Kang SG, Kim SH, Moon JH, Kim EH, et al. Survival, prognostic factors, and volumetric analysis of extent of resection for anaplastic gliomas. Cancer Res Treat. 2020; 52:1041–1049. PMID:
32324987.
29. Pessina F, Navarria P, Cozzi L, Ascolese AM, Simonelli M, Santoro A, et al. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? A single institution retrospective experience. J Neurooncol. 2017; 135:129–139. PMID:
28689368.
30. Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg. 2016; 124:977–988. PMID:
26495941.
31. Incekara F, Koene S, Vincent AJPE, van den Bent MJ, Smits M. Association between supratotal glioblastoma resection and patient survival: a systematic review and meta-analysis. World Neurosurg. 2019; 127:617–624.e2. PMID:
31004858.
32. Jackson C, Choi J, Khalafallah AM, Price C, Bettegowda C, Lim M, et al. A systematic review and meta-analysis of supratotal versus gross total resection for glioblastoma. J Neurooncol. 2020; 148:419–431. PMID:
32562247.
33. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006; 7:392–401. PMID:
16648043.
34. Eatz TA, Eichberg DG, Lu VM, Di L, Komotar RJ, Ivan ME. Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: a systematic review. J Neurooncol. 2022; 156:233–256. PMID:
34989964.
35. Roh TH, Kang SG, Moon JH, Sung KS, Park HH, Kim SH, et al. Survival benefit of lobectomy over gross-total resection without lobectomy in cases of glioblastoma in the noneloquent area: a retrospective study. J Neurosurg. 2019; 132:895–901. PMID:
30835701.
36. Schneider M, Potthoff AL, Keil VC, Güresir Á, Weller J, Borger V, et al. Surgery for temporal glioblastoma: lobectomy outranks oncosurgical-based gross-total resection. J Neurooncol. 2019; 145:143–150. PMID:
31485921.
37. Shah AH, Mahavadi A, Di L, Sanjurjo A, Eichberg DG, Borowy V, et al. Survival benefit of lobectomy for glioblastoma: moving towards radical supramaximal resection. J Neurooncol. 2020; 148:501–508. PMID:
32627128.
38. Baik SH, Kim SY, Na YC, Cho JM. Supratotal resection of glioblastoma: better survival outcome than gross total resection. J Pers Med. 2023; 13:383. PMID:
36983564.
39. Gerritsen JKW, Zwarthoed RH, Kilgallon JL, Nawabi NL, Jessurun CAC, Versyck G, et al. Effect of awake craniotomy in glioblastoma in eloquent areas (GLIOMAP): a propensity score-matched analysis of an international, multicentre, cohort study. Lancet Oncol. 2022; 23:802–817. PMID:
35569489.
40. Gerritsen JKW, Zwarthoed RH, Kilgallon JL, Nawabi NL, Versyck G, Jessurun CAC, et al. Impact of maximal extent of resection on postoperative deficits, patient functioning, and survival within clinically important glioblastoma subgroups. Neuro Oncol. 2023; 25:958–972. PMID:
36420703.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download