Abstract
Pituitary apoplexy (PA) is a clinical syndrome resulting from sudden hemorrhage and/or infarction of the pituitary gland. Recent reports documented the development of PA secondary to treatment with gonadotropin-releasing hormone (GnRH) agonists for prostate cancer. A 52-year-old woman visited our emergency room with a severe headache, occurred 1 day prior. She underwent breast-conserving surgery for breast cancer 1 month prior. She was currently undergoing radiation and hormone therapy, consisting of leuprorelin. Brain contrast-enhanced MRI revealed a pituitary adenoma with internal hemorrhage in the sellar and suprasellar areas. Pachymeningeal enhancement was observed along the retroclival and bilateral frontal areas. The patient was diagnosed with PA and aseptic meningitis. The patient underwent total excision via transsphenoidal surgery 8 days after admission. The patient was pathologically diagnosed with a pituitary adenoma with necrosis. On immunochemical staining, the tumor was positive for follicle-stimulating hormone. The follow-up MRI revealed no evidence of residual tumor or an improved pachymeningeal enhancement. She is currently undergoing follow-up at the neurosurgery and endocrinology outpatient departments with no noted complications. In breast cancer patients receiving GnRH agonist therapy, PA may be rare complication.
Pituitary apoplexy (PA) is a clinical syndrome, resulting from sudden hemorrhage and/or infarction of the pituitary gland. It clinically presents as headaches or visual disturbances, so it is necessary to differentiate it from diseases, such as a subarachnoid hemorrhage or bacterial meningitis [1]. It is a rare disease with 6.2 cases per 100,000 individuals [2]. However, early recognition of this disease remains essential because it is fatal. Therefore, it is crucial to investigate predisposing factors, including trauma, bromocriptine, anticoagulation, and angiographic procedures [13]. Recent reports have documented the development of PA among prostate cancer patients receiving gonadotropin-releasing hormone (GnRH) agonist therapy [45]. This study reports a rare case of PA in a breast cancer patient, treated via GnRH agonist therapy.
A 52-year-old perimenopausal woman visited our emergency room due to a severe headache, occurred 1 day prior. She complained of nausea, vomiting, and left-sided visual disturbances. She has been taking oral steroid pills and 2 tablets of prednisolone (Solondo Tab 5 mg; Yuhan Corp., Seoul, Korea) for asthma daily since September 2021. She underwent breast-conserving surgery for breast cancer at our institution 1 month prior. She was pathologically diagnosed with triple-positive invasive ductal carcinoma. She currently underwent radiotherapy and hormonal therapy, consisting of leuprorelin. Her first dose of leuprorelin (3.75 mg) was subcutaneously injected 20 days prior. She was alert and afebrile with stable blood pressure and respiratory rate. On physical examination, the patient had full and equal extraocular movements without visual field defects. The remaining neurological examinations yielded essentially normal results. At that time, her headache was conservatively treated with metoclopramide and acetaminophen. A brain contrast-enhanced MRI was performed 2 days after admission. A 1.8-cm T2 heterogeneous signal intensity lesion was observed in the sellar and suprasellar areas with intralesional T1 hypersignal intensity material. On post-contrast T1-weighed imaging, an intralesional contrast-enhanced portion was noted in the left lateral aspect of the lesion with pachymeningeal enhancement along the retroclival and bilateral frontal areas. The pituitary stalk deviated to the right side, and the optic chiasm was displaced superiorly by the mass (Fig. 1). On laboratory evaluation, she had a follicle-stimulating hormone (FSH) of 3.9 mIU/mL, luteinizing hormone (LH) of 0.4 mIU/mL, and estradiol of 41 pg/mL. The other endocrinological laboratory results are summarized in Table 1. The patient was clinically diagnosed with PA and underwent transsphenoidal surgery (TSA) 8 days after administration. The tumor on the left sellar area was encapsulated with hemorrhagic and necrotic contents. A total excision was performed, but the left sellar capsule was intact. The patient was pathologically diagnosed with a pituitary adenoma with necrosis. The immunochemical staining was positive for FSH, non-specific for LH (Fig. 2), and weakly positive for prolactin. The postoperative MRI showed no evidence of a residual tumor, and the patient was discharged 14 days postoperatively. Improvement of the pachymeningeal enhancement was noted on the follow-up MRI 3 months after surgery (Fig. 3). At the 6 months follow-up, the patient has remained well without complications and is undergoing follow-up at the neurosurgery and endocrinology outpatient clinics.
This report documents a PA case of a GnRH-releasing pituitary adenoma in a breast cancer patient treated with leuprolide. First reported in 1995, PA has been reported in more than 20 patients treated with GnRH agonists [467]. Most cases involved prostate cancer patients, who were treated with GnRH agonists. Meanwhile, a few cases involving breast cancer patients have been reported (Table 2) [568]. The common time of onset was within 4 hours; however, one reported case of PA was detected 1 year after the administration of the final last dose [6]. According to a systemic review reported in 2021, among 12 prostate cancer patients with GnRH agonist-related PA who had undergone surgical treatment, 10 had tumors positive for LH and FSH on immunohistochemistry, while 2 were positive for FSH only, like the patient in the present case [4].
Although the mechanism behind the development of PA in patients treated with GnRH agonists remains vague, several hypotheses have been proposed. These included increased tumor vascularity [4] and a mismatch in blood supply [9]. Since it occurs during the acute and subacute phases, a dual pathology was also proposed. The acute phase, which occurs within minutes or hours after the administration of a GnRH agonist, reportedly resulted from metabolic hyperactivity and cell degranulation/shrinking in adenomas with reduced perfusion. However, in the subacute phase, LH secretion increases tumor size and causes ischemia/hemorrhage [7].
Breast cancer is one of the most common cancers in women. According to global cancer statistics in 2018, 2.1 million new cases were diagnosed worldwide [10]. GnRH agonists, such as leuprolide, are administered to patients with hormone receptor-positive early breast cancer. It lowers the risk of recurrence after treatment [5]. Recent reports have discussed the preservation of ovarian function in breast cancer patients undergoing chemotherapy [11]. Recent studies have aimed to improve the early diagnosis of breast cancer. Consequently, the development of PA has been identified as a complication of GnRH agonist treatment. This should be considered when selecting the treatment for early breast cancer patients.
MRI is an important diagnostic modality for the detection of pituitary adenomas. In prostate cancer patients treated with GnRH agonists, performing an MRI to screen for the presence of pituitary adenomas prior to GnRH treatment is not recommended due to its cost [4]. Breast cancer is the second most common cancer that causes brain metastasis after lung cancer [12]. Although brain MRIs are not routinely performed for metastasis screening for all breast cancer patients, recent studies have shown that MRI screening is helpful for high-risk patients [13]. Therefore, in patients receiving GnRH agonist treatment, the development of PA should be evaluated because it is a life-threatening complication. Thus, immediate brain MRI is recommended for patients with sudden-onset headaches and those undergoing GnRH agonist treatment. In patients with a previous brain MRI, the imaging results should be reviewed.
In this case, pachymeningeal enhancement was observed in the retroclival area and bilateral frontal convexity on MRI. Pachymeningeal enhancement occurs in benign conditions, including intracranial hypotension or postoperative changes, and malignant conditions, such as metastatic disease [14]. In particular, the frequency of isolated pachymeningeal metastasis in metastatic breast cancer was reportedly 1.5%. Pituitary abscesses may also present with clinical and imaging findings similar to PA [1516]. Furthermore, it is difficult to exclude infectious conditions in PA patients because the cerebrospinal fluid (CSF) study may not be helpful due to the accompanying chemical meningitis [17]. In this case, the pachymeningeal enhancement completely improved on follow-up MRI. This imaging finding has been reported in PA patients; therefore, a PA was considered more likely in the present case. In breast cancer patients presenting with sudden-onset headache and pachymeningeal enhancement on MRI, the differential diagnosis includes dural metastasis, infection, and intracranial hypotension. Moreover, in patients with a history of GnRH agonist treatment, PA with aseptic meningitis secondary to GnRH agonist treatment should be considered. Invasive tests, such as a CSF study, may be omitted to establish the treatment plan.
PA is a rare but potentially fatal condition that can occur in breast cancer patients who have received GnRH agonist treatment. Thus, patients should be informed of this complication. Moreover, it should be considered in symptomatic patients for early diagnosis and treatment.
Acknowledgments
The authors are grateful to Professor Hee Kyung Kim (Department of Pathology, Soonchunhyang University Bucheon Hospital) for her pathological analysis and advice.
Notes
Ethics Statement: The study conformed to the ethical guidelines of the World Medical Association’s 1975 Declaration of Helsinki and was approved by the Soonchunhyang University Hospital Institutional Review Board (IRB). The need for informed consent was waived by the IRB (IRB No. SCHBC 2022-11-032).
Availability of Data and Material
The data that support the findings of this study are available on request from the corresponding author.
References
1. Briet C, Salenave S, Bonneville JF, Laws ER, Chanson P. Pituitary apoplexy. Endocr Rev. 2015; 36:622–645. PMID: 26414232.
2. Fernandez A, Karavitaki N, Wass JA. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf). 2010; 72:377–382. PMID: 19650784.
3. Laws ER. Pituitary tumor apoplexy: a review. J Intensive Care Med. 2008; 23:146–147. PMID: 18372353.
4. Raj R, Elshimy G, Jacob A, Arya PVA, Unnikrishnan DC, Correa R, et al. Pituitary apoplexy induced by gonadotropin-releasing hormone (GnRH) agonist administration for treatment of prostate cancer: a systematic review. J Cancer Res Clin Oncol. 2021; 147:2337–2347. PMID: 34156518.
5. Tanios G, Mungo NA, Kapila A, Bajaj K. Pituitary apoplexy: a rare complication of leuprolide therapy in prostate cancer treatment. BMJ Case Rep. 2017; 2017:bcr2016218514.
6. Elshimy G, Raj R, Jacob A, Correa R. Late onset of pituitary apoplexy following gonadotropin-releasing hormone agonist for prostate cancer treatment. BMJ Case Rep. 2022; 15:e248523.
7. Barbosa M, Paredes S, Machado MJ, Almeida R, Marques O. Pituitary apoplexy induced by gonadotropin-releasing hormone agonist administration: a rare complication of prostate cancer treatment. Endocrinol Diabetes Metab Case Rep. 2020; 2020:20-0018.
8. Voica M, Tetlay M, Hasan F. Abstract# 993208: pituitary apoplexy secondary to GnRH agonist, leuprolide, for breast cancer: a case study and review of the literature. Endocr Pract. 2021; 27(supplement):S108–S109.
9. Sasagawa Y, Tachibana O, Nakagawa A, Koya D, Iizuka H. Pituitary apoplexy following gonadotropin-releasing hormone agonist administration with gonadotropin-secreting pituitary adenoma. J Clin Neurosci. 2015; 22:601–603. PMID: 25455737.
10. Chen Z, Xu L, Shi W, Zeng F, Zhuo R, Hao X, et al. Trends of female and male breast cancer incidence at the global, regional, and national levels, 1990-2017. Breast Cancer Res Treat. 2020; 180:481–490. PMID: 32056055.
11. Poggio F, Lambertini M, Bighin C, Conte B, Blondeaux E, D’Alonzo A, et al. Potential mechanisms of ovarian protection with gonadotropin-releasing hormone agonist in breast cancer patients: a review. Clin Med Insights Reprod Health. 2019; 13:1179558119864584. PMID: 31391786.
12. Berghoff AS, Schur S, Fureder LM, Gatterbauer B, Dieckmann K, Widhalm G, et al. Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open. 2016; 1:e000024. PMID: 27843591.
13. Hadjipanteli A, Doolan P, Kyriacou E, Constantinidou A. Breast cancer brain metastasis: the potential role of MRI beyond current clinical applications. Cancer Manag Res. 2020; 12:9953–9964. PMID: 33116852.
14. Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007; 27:525–551. PMID: 17374867.
15. Wolansky LJ, Gallagher JD, Heary RF, Malantic GP, Dasmahapatra A, Shaderowfsky PD, et al. MRI of pituitary abscess: two cases and review of the literature. Neuroradiology. 1997; 39:499–503. PMID: 9258927.
16. Colli ML, Migowski W Jr, Czepielewski MA, Ferreira N, Gross JL. [Pituitary abscess simulating apoplexy]. Arq Bras Endocrinol Metabol. 2006; 50:1122–1126. Portuguese. PMID: 17221121.
17. Bjerre P, Lindholm J. Pituitary apoplexy with sterile meningitis. Acta Neurol Scand. 1986; 74:304–307. PMID: 3811836.
Fig. 1
Initial MRI (A: Coronal T2, B: coronal precontrast T1, C-F: coronal contrast-enhanced-T1, G: sagittal contrast-enhanced-T1). A and B: The lesion showing heterogeneous signal intensity on T2 and high signal intensity on T1 was noted in the sellar and suprasellar regions. C: The left lateral aspect of the lesion exhibited gadolinium enhancement. D: The pituitary stalk was deviated on the right side, and the optic chiasm bowed upwardly. E and F: The pachymeningeal enhancement is accompanied in the retroclival area and bilateral frontal convexities.
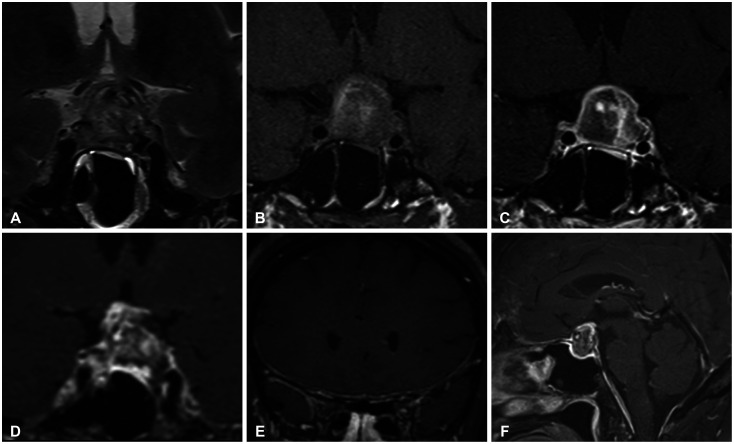
Fig. 2
Pituitary hormones analyzed by immunochemistry in pituitary adenoma. The pituitary adenoma cells showed positive immunostaining for follicle-stimulating hormone (A, ×200), but non-specific for luteinising hormone (B, ×200).
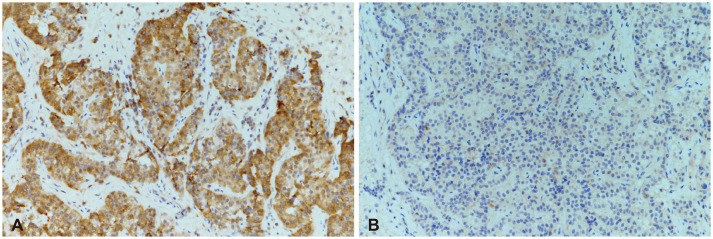
Fig. 3
Follow-up MRI, 3 months postoperatively. The previously noted pituitary adenoma in the sellar and suprasellar regions was removed (A and B), and pachymeningeal enhancement also improved (C and D).
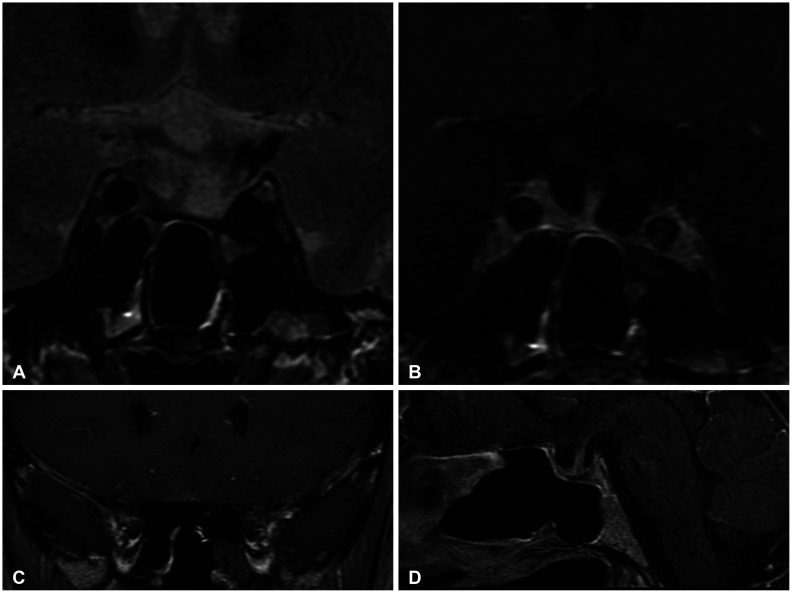
Table 1
Laboratory test at admission (after steroid)
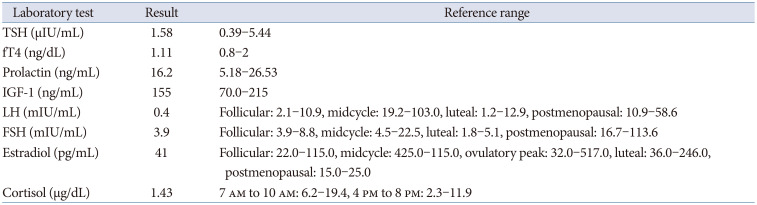
Table 2
Summary of reported PA cases in breast cancer treated with GnRH agonist
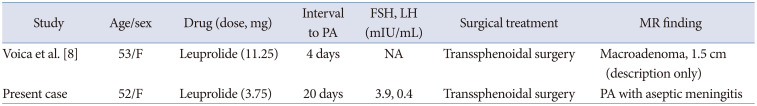
| Study | Age/sex | Drug (dose, mg) | Interval to PA | FSH, LH (mIU/mL) | Surgical treatment | MR finding |
|---|---|---|---|---|---|---|
| Voica et al. [8] | 53/F | Leuprolide (11.25) | 4 days | NA | Transsphenoidal surgery | Macroadenoma, 1.5 cm (description only) |
| Present case | 52/F | Leuprolide (3.75) | 20 days | 3.9, 0.4 | Transsphenoidal surgery | PA with aseptic meningitis |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download