INTRODUCTION
CASE REPORT
Fig. 1
Representative T2/fluid-attenuated inversion recovery MRI on admission (axial, coronal, and sagittal, from left to right, respectively). These images show a hyperintense non-enhancing mass in the left temporal lobe, measuring 4.1×3.6×3.4 cm with extension to posterior superior temporal gyrus (arrow).
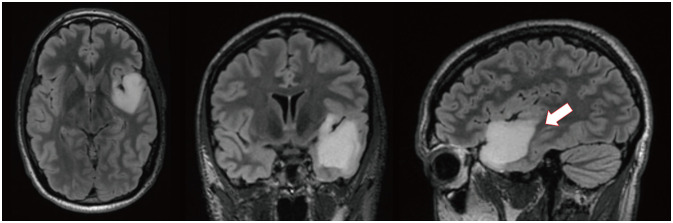
Fig. 2
Functional magnetic resonance imaging (fMRI). A: Axial fMRI shows activation of language areas during word-generation/sentence completion tasks in bilateral cerebral hemispheres in temporal lobes (left greater than right; arrowhead). B: Axial fMRI shows blood-oxygen-level dependent activation can be seen superior to the mass during the movement of the tongue in the precentral gyrus bilaterally (arrowhead).
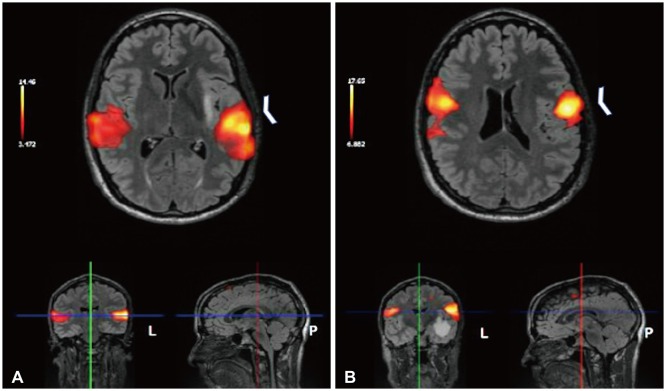
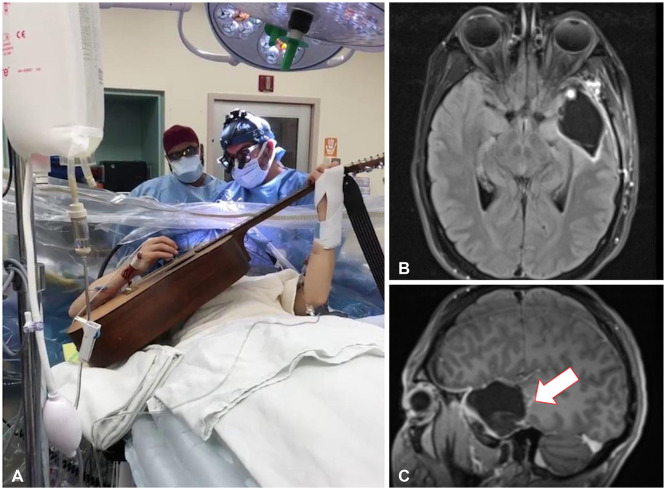
Fig. 4
Resection of brain tumor with speech mapping. A: Intraoperative image of the patient playing his guitar during the awake craniotomy. B and C: Postoperative axial (B) and sagittal (C) fluid-attenuated inversion recovery MRI showing resection cavity with a small residual portion of blood products and granulation tissue at the base (arrow).
DISCUSSION
Table 1
Awake craniotomy for music mapping in brain tumor resection: demographic, clinical, and operative characteristics for cases with standard music tasks
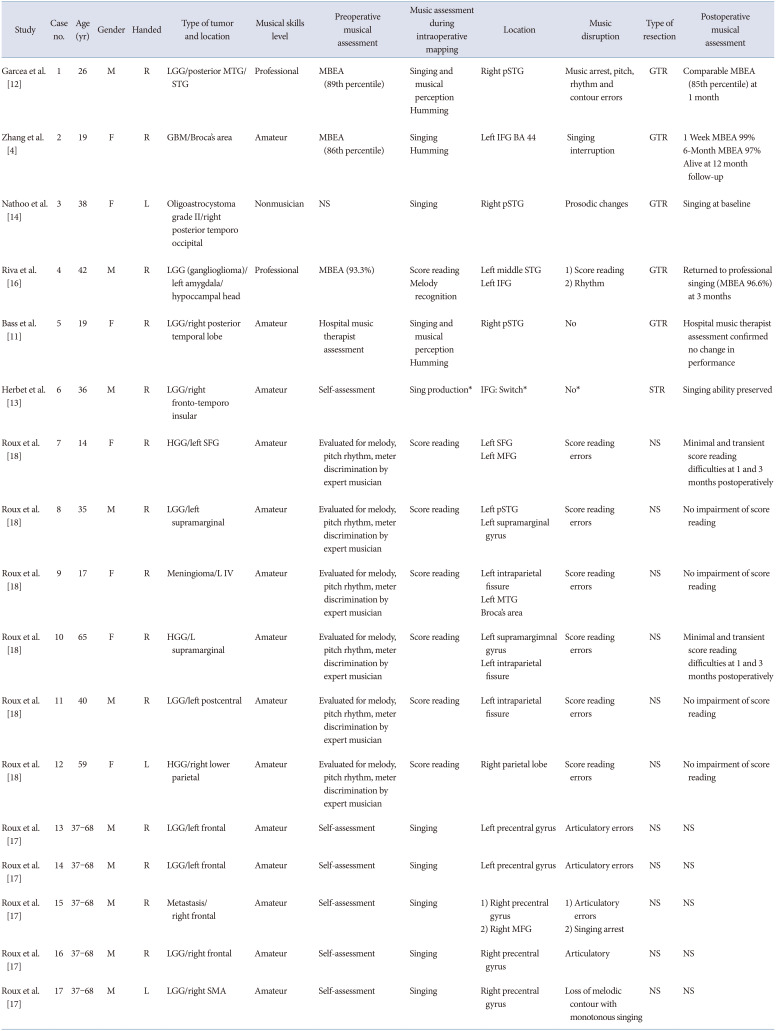
| Study | Case no. | Age (yr) | Gender | Handed | Type of tumor and location | Musical skills level | Preoperative musical assessment | Music assessment during intraoperative mapping | Location | Music disruption | Type of resection | Postoperative musical assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Garcea et al. [12] | 1 | 26 | M | R | LGG/posterior MTG/STG | Professional | MBEA (89th percentile) |
Singing and musical perception Humming |
Right pSTG | Music arrest, pitch, rhythm and contour errors | GTR | Comparable MBEA (85th percentile) at 1 month |
| Zhang et al. [4] | 2 | 19 | F | R | GBM/Broca’s area | Amateur | MBEA (86th percentile) |
Singing Humming |
Left IFG BA 44 | Singing interruption | GTR |
1 Week MBEA 99% 6-Month MBEA 97% Alive at 12 month follow-up |
| Nathoo et al. [14] | 3 | 38 | F | L | Oligoastrocystoma grade II/right posterior temporo occipital | Nonmusician | NS | Singing | Right pSTG | Prosodic changes | GTR | Singing at baseline |
| Riva et al. [16] | 4 | 42 | M | R | LGG (ganglioglioma)/left amygdala/hypoccampal head | Professional | MBEA (93.3%) |
Score reading Melody recognition |
Left middle STG Left IFG |
1) Score reading 2) Rhythm |
GTR | Returned to professional singing (MBEA 96.6%) at 3 months |
| Bass et al. [11] | 5 | 19 | F | R | LGG/right posterior temporal lobe | Amateur | Hospital music therapist assessment |
Singing and musical perception Humming |
Right pSTG | No | GTR | Hospital music therapist assessment confirmed no change in performance |
| Herbet et al. [13] | 6 | 36 | M | R | LGG/right fronto-temporo insular | Amateur | Self-assessment | Sing production* | IFG: Switch* | No* | STR | Singing ability preserved |
| Roux et al. [18] | 7 | 14 | F | R | HGG/left SFG | Amateur | Evaluated for melody, pitch rhythm, meter discrimination by expert musician | Score reading |
Left SFG Left MFG |
Score reading errors | NS | Minimal and transient score reading difficulties at 1 and 3 months postoperatively |
| Roux et al. [18] | 8 | 35 | M | R | LGG/left supramarginal | Amateur | Evaluated for melody, pitch rhythm, meter discrimination by expert musician | Score reading |
Left pSTG Left supramarginal |
Score reading errors | NS | No impairment of score reading |
| Roux et al. [18] | 9 | 17 | F | R | Meningioma/L IV | Amateur | Evaluated for melody, pitch rhythm, meter discrimination by expert musician | Score reading |
Left intraparietal fissure Left MTG Broca’s area |
Score reading errors | NS | No impairment of score reading |
| Roux et al. [18] | 10 | 65 | F | R | HGG/L supramarginal | Amateur | Evaluated for melody, pitch rhythm, meter discrimination by expert musician | Score reading |
Left supramargimnal gyrus Left intraparietal fissure |
Score reading errors | NS | Minimal and transient score reading difficulties at 1 and 3 months postoperatively |
| Roux et al. [18] | 11 | 40 | M | R | LGG/left postcentral | Amateur | Evaluated for melody, pitch rhythm, meter discrimination by expert musician | Score reading | Left intraparietal fissure | Score reading errors | NS | No impairment of score reading |
| Roux et al. [18] | 12 | 59 | F | L | HGG/right lower parietal | Amateur | Evaluated for melody, pitch rhythm, meter discrimination by expert musician | Score reading | Right parietal lobe | Score reading errors | NS | No impairment of score reading |
| Roux et al. [17] | 13 | 37–68 | M | R | LGG/left frontal | Amateur | Self-assessment | Singing | Left precentral gyrus | Articulatory errors | NS | NS |
| Roux et al. [17] | 14 | 37–68 | M | R | LGG/left frontal | Amateur | Self-assessment | Singing | Left precentral gyrus | Articulatory errors | NS | NS |
| Roux et al. [17] | 15 | 37–68 | M | R | Metastasis/right frontal | Amateur | Self-assessment | Singing |
1) Right precentral gyrus 2) Singing arrest |
1) Articulatory errors 2) Right MFG |
NS | NS |
| Roux et al. [17] | 16 | 37–68 | M | R | LGG/right frontal | Amateur | Self-assessment | Singing | Right precentral gyrus | Articulatory | NS | NS |
| Roux et al. [17] | 17 | 37–68 | M | L | LGG/right SMA | Amateur | Self-assessment | Singing | Right precentral gyrus | Loss of melodic contour with monotonous singing | NS | NS |
*Switch from normal speech to singing. LGG, low grade glioma; MTG, middle temporal gyrus; STG, superior temporal gyrus; MBEA, Montreal Battery of Evaluation of Amusia; pSTG, posterior superior temporal gyrus; GTR, gross total resection; GBM, glioblastoma multiforme; IFG; inferior frontal gyrus; BA, Brodmann area; NS, non-specified; HGG, high grade glioma; SFG, superior frontal gyrus; MFG, middle frontal gyrus; SMA, supplementary motor area; STR, subtotal resection; NTR, near total resection
Table 2
Cases of awake craniotomy and intraoperative instrument playing: demographic, clinical, and operative characteristics
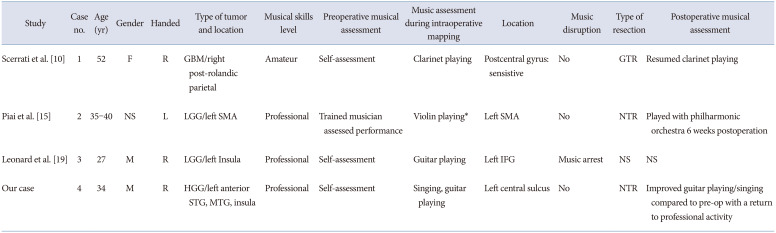
| Study | Case no. | Age (yr) | Gender | Handed | Type of tumor and location | Musical skills level | Preoperative musical assessment | Music assessment during intraoperative mapping | Location | Music disruption | Type of resection | Postoperative musical assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scerrati et al. [10] | 1 | 52 | F | R | GBM/right post-rolandic parietal | Amateur | Self-assessment | Clarinet playing | Postcentral gyrus: sensistive | No | GTR | Resumed clarinet playing |
| Piai et al. [15] | 2 | 35–40 | NS | L | LGG/left SMA | Professional | Trained musician assessed performance | Violin playing* | Left SMA | No | NTR | Played with philharmonic orchestra 6 weeks postoperation |
| Leonard et al. [19] | 3 | 27 | M | R | LGG/left Insula | Professional | Self-assessment | Guitar playing | Left IFG | Music arrest | NS | NS |
| Our case | 4 | 34 | M | R | HGG/left anterior STG, MTG, insula | Professional | Self-assessment | Singing, guitar playing | Left central sulcus | No | NTR | Improved guitar playing/singing compared to pre-op with a return to professional activity |
*Seizure arrested intraoperative assessment. GBM, glioblastoma multiforme; GTR, gross total resection; LGG, low grade glioma; SMA, supplementary motor area; NTR, near total resection; IFG, inferior frontal gyrus; NS, non-specified; HGG, high grade glioma; STG, superior temporal gyrus; MTG, middle temporal gyrus




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download