Abstract
Background
Methods
Results
Notes
Author Contributions:
Conceptualization: Charles E. Mackel, Rafael A. Vega, Ron L. Alterman.
Data curation: Charles E. Mackel.
Formal analysis: Charles E. Mackel.
Investigation: Charles E. Mackel, Harry Rosenberg, Hemant Varma.
Methodology: Charles E. Mackel.
Resources: Hemant Varma.
Supervision: Rafael A. Vega, Ron L. Alterman.
Visualization: Harry Rosenberg.
Writing—original draft: Charles E. Mackel.
Writing—review & editing: Charles E. Mackel, Harry Rosenberg, Erik J. Uhlmann, Rafael A. Vega, Ron L. Alterman.
Availability of Data and Material
References
Fig. 1
Axial fat-suppressed post-gadolinium of the calf. Soft tissue hemangioma (arrow) in the lateral head of the left gastrocnemius in the presence of enchondromas consistent with Maffucci syndrome.
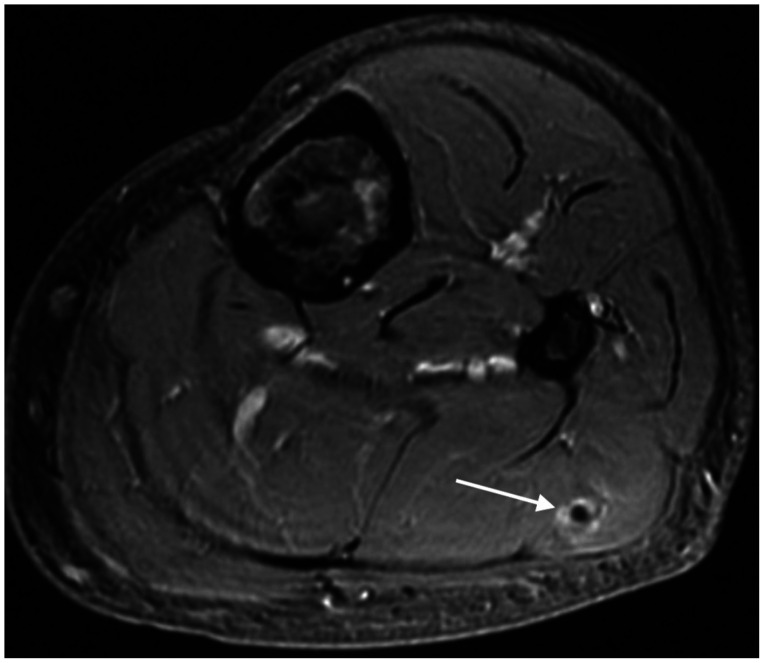
Fig. 2
Imaging of the left distal femur over time, demonstrating a soft tissue mass with cortical destruction. A: Left femoral enchondroma on axial CT 2 years prior to malignant conversion with avidity of 2.6 on associated FDG-PET. B: Same femoral enchondroma on axial CT at time of dedifferentiation demonstrating cortical thinning and breakthrough. C: FDG-PET avidity in femoral chondrosarcoma increased to 17.6. Remainder of body scan negative.
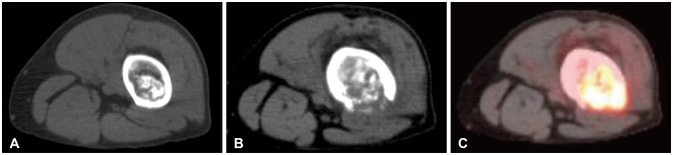
Fig. 3
Axial T1 post-gadolinium MRI demonstrating heterogeneously enhancing, intra-axial right parietal brain metastasis and surrounding vasogenic edema 4 months after primary diagnosis.
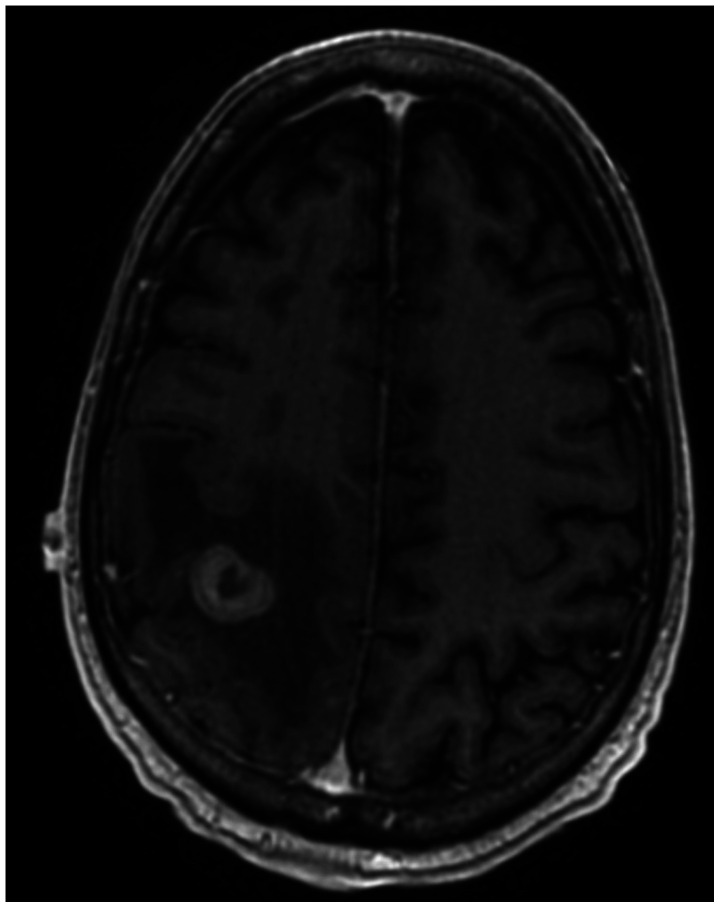
Fig. 4
Histochemistry of malignant tissue from femoral (A and B) and cerebral (C) tumor sites (hematoxylin and eosin stain). A: At low power (×10), high-grade chondrosarcoma on the right with areas of tumor necrosis on the left. B: Higher power (×20) highlights striking nuclear pleomorphism, prominent nucleoli, and mitotic activity. C: Malignant neoplasm in the brain (×20) with similar morphological features as femoral biopsy and nuclear pleomorphism, prominent nucleoli, abundant mitoses.
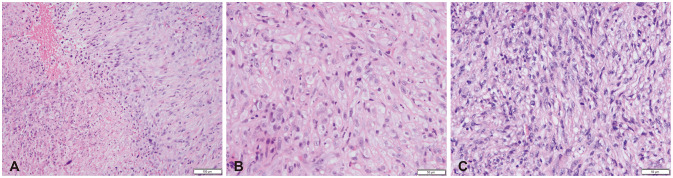
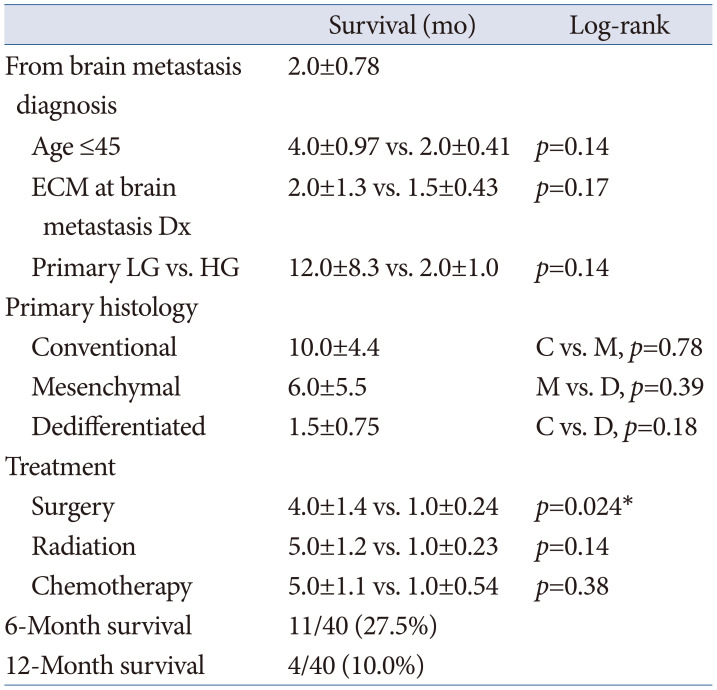
Fig. 6
Survival following extracranial chondrosarcoma metastatic to brain. A: Kaplan-Meier survival graph for chondrosarcomas following brain metastasis. B: Comparison of survival between patients with surgical resection versus non-surgical management of brain metastases with significantly different survival outcomes on log-rank test (p=0.024).
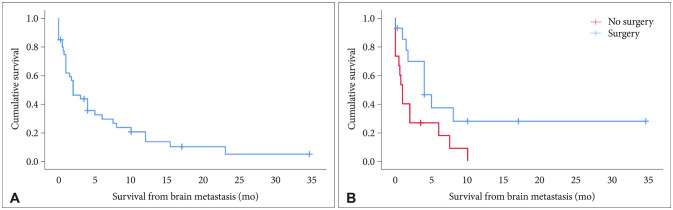
Table 1
Compilation of clinical characteristics of patients who subsequently developed chondrosarcoma brain metastases
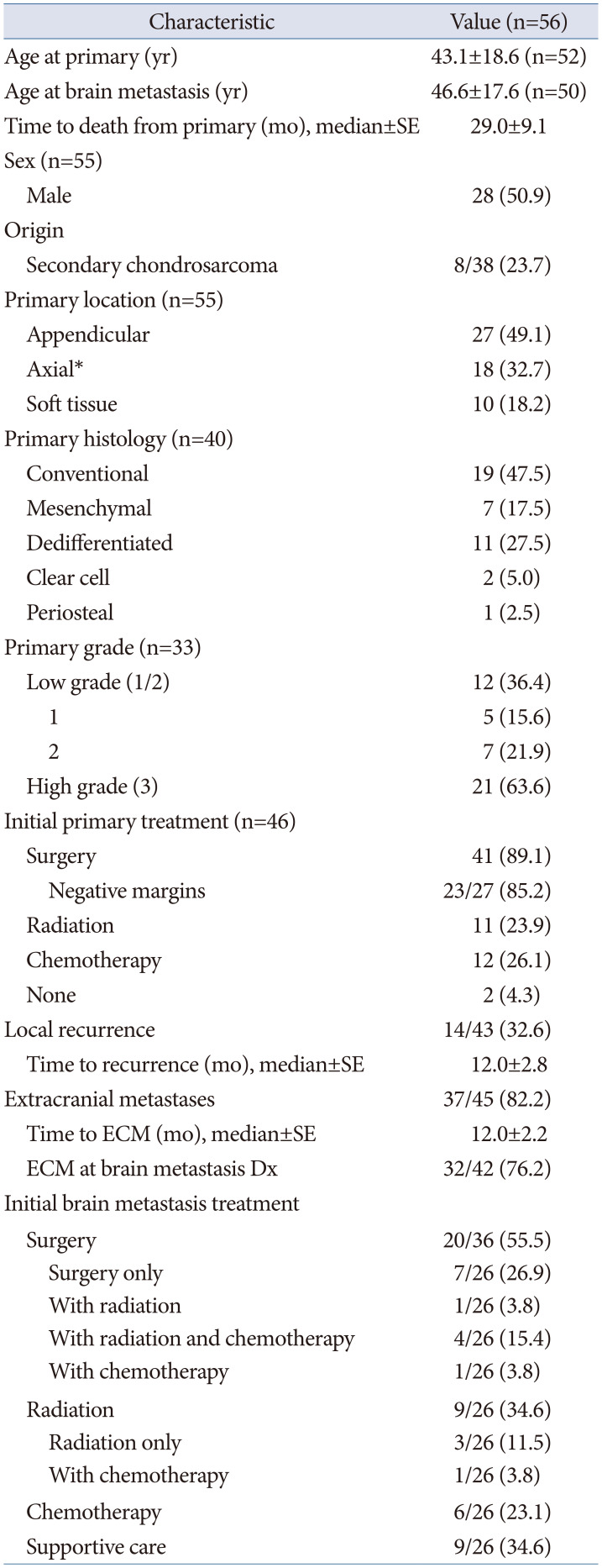
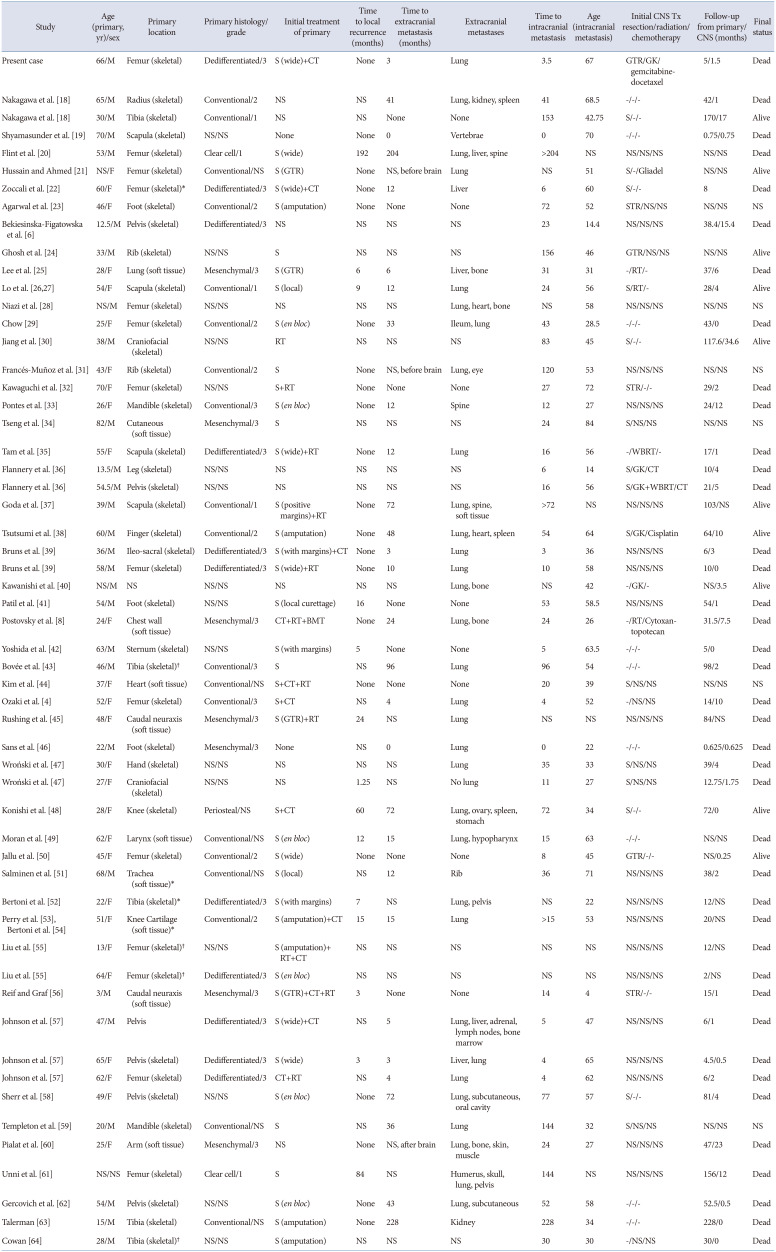
Table 2
Literature review of clinical characteristics of patients with cerebral chondrosarcoma metastasis
| Study | Age (primary, yr)/sex | Primary location | Primary histology/grade | Initial treatment of primary | Time to local recurrence (months) | Time to extracranial metastasis (months) | Extracranial metastases | Time to intracranial metastasis | Age (intracranial metastasis) | Initial CNS Tx resection/radiation/chemotherapy | Follow-up from primary/CNS (months) | Final status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present case | 66/M | Femur (skeletal) | Dedifferentiated/3 | S (wide)+CT | None | 3 | Lung | 3.5 | 67 | GTR/GK/gemcitabine-docetaxel | 5/1.5 | Dead |
| Nakagawa et al. [18] | 65/M | Radius (skeletal) | Conventional/2 | NS | NS | 41 | Lung, kidney, spleen | 41 | 68.5 | -/-/- | 42/1 | Dead |
| Nakagawa et al. [18] | 30/M | Tibia (skeletal) | Conventional/1 | NS | NS | None | None | 153 | 42.75 | S/-/- | 170/17 | Alive |
| Shyamasunder et al. [19] | 70/M | Scapula (skeletal) | NS/NS | None | None | 0 | Vertebrae | 0 | 70 | -/-/- | 0.75/0.75 | Dead |
| Flint et al. [20] | 53/M | Femur (skeletal) | Clear cell/1 | S (wide) | 192 | 204 | Lung, liver, spine | >204 | NS | NS/NS/NS | NS/NS | Dead |
| Hussain and Ahmed [21] | NS/F | Femur (skeletal) | Conventional/NS | S (GTR) | None | NS, before brain | Lung | NS | 51 | S/-/Gliadel | NS/NS | Alive |
| Zoccali et al. [22] | 60/F | Femur (skeletal)* | Dedifferentiated/3 | S (wide)+CT | None | 12 | Liver | 6 | 60 | S/-/- | 8.0 | Dead |
| Agarwal et al. [23] | 46/F | Foot (skeletal) | Conventional/2 | S (amputation) | None | None | None | 72 | 52 | STR/NS/NS | NS/NS | NS |
| Bekiesinska-Figatowska et al. [6] | 12.5/M | Pelvis (skeletal) | Dedifferentiated/3 | NS | NS | NS | NS | 23 | 14.4 | NS/NS/NS | 38.4/15.4 | Dead |
| Ghosh et al. [24] | 33/M | Rib (skeletal) | NS/NS | S | NS | NS | NS | 156 | 46 | GTR/NS/NS | NS/NS | Alive |
| Lee et al. [25] | 28/F | Lung (soft tissue) | Mesenchymal/3 | S (GTR) | 6 | 6 | Liver, bone | 31 | 31 | -/RT/- | 37/6 | Dead |
| Lo et al. [2627] | 54/F | Scapula (skeletal) | Conventional/1 | S (local) | 9 | 12 | Lung | 24 | 56 | S/RT/- | 28/4 | Alive |
| Niazi et al. [28] | NS/M | Femur (skeletal) | NS/NS | NS | NS | NS | Lung, heart, bone | NS | 58 | NS/NS/NS | NS/NS | NS |
| Chow [29] | 25/F | Femur (skeletal) | Conventional/2 | S (en bloc) | None | 33 | Ileum, lung | 43 | 28.5 | -/-/- | 43/0 | Dead |
| Jiang et al. [30] | 38/M | Craniofacial (skeletal) | NS/NS | RT | NS | NS | NS | 83 | 45 | S/-/- | 117.6/34.6 | Alive |
| Francés-Muñoz et al. [31] | 43/F | Rib (skeletal) | Conventional/2 | S | None | NS, before brain | Lung, eye | 120 | 53 | NS/NS/NS | NS/NS | NS |
| Kawaguchi et al. [32] | 70/F | Femur (skeletal) | NS/NS | S+RT | None | None | None | 27 | 72 | STR/-/- | 29/2 | Dead |
| Pontes et al. [33] | 26/F | Mandible (skeletal) | Conventional/3 | S (en bloc) | None | 12 | Spine | 12 | 27 | NS/NS/NS | 24/12 | Dead |
| Tseng et al. [34] | 82/M | Cutaneous (soft tissue) | Mesenchymal/3 | S | NS | NS | NS | 24 | 84 | S/NS/NS | NS/NS | NS |
| Tam et al. [35] | 55/F | Scapula (skeletal) | Dedifferentiated/3 | S (wide)+RT | None | 12 | Lung | 16 | 56 | -/WBRT/- | 17/1 | Dead |
| Flannery et al. [36] | 13.5/M | Leg (skeletal) | NS/NS | NS | NS | NS | NS | 6 | 14 | S/GK/CT | 10/4 | Dead |
| Flannery et al. [36] | 54.5/M | Pelvis (skeletal) | NS/NS | NS | NS | NS | NS | 16 | 56 | S/GK+WBRT/CT | 21/5 | Dead |
| Goda et al. [37] | 39/M | Scapula (skeletal) | Conventional/1 | S (positive margins)+RT | None | 72 | Lung, spine, soft tissue | >72 | NS | NS/NS/NS | 103/NS | Alive |
| Tsutsumi et al. [38] | 60/M | Finger (skeletal) | Conventional/2 | S (amputation) | None | 48 | Lung, heart, spleen | 54 | 64 | S/GK/Cisplatin | 64/10 | Alive |
| Bruns et al. [39] | 36/M | Ileo-sacral (skeletal) | Dedifferentiated/3 | S (with margins)+CT | None | 3 | Lung | 3 | 36 | NS/NS/NS | 6/3 | Dead |
| Bruns et al. [39] | 58/M | Femur (skeletal) | Dedifferentiated/3 | S (wide)+RT | None | 10 | Lung | 10 | 58 | NS/NS/NS | 10/0 | Dead |
| Kawanishi et al. [40] | NS/M | NS | NS/NS | NS | NS | NS | Lung, bone | NS | 42 | -/GK/- | NS/3.5 | Alive |
| Patil et al. [41] | 54/M | Foot (skeletal) | NS/NS | S (local curettage) | 16 | None | None | 53 | 58.5 | NS/NS/NS | 54/1 | Dead |
| Postovsky et al. [8] | 24/F | Chest wall (soft tissue) | Mesenchymal/3 | CT+RT+BMT | None | 24 | Lung, bone | 24 | 26 | -/RT/Cytoxan-topotecan | 31.5/7.5 | Dead |
| Yoshida et al. [42] | 63/M | Sternum (skeletal) | NS/NS | S (with margins) | 5 | None | None | 5 | 63.5 | -/-/- | 5/0 | Dead |
| Bovée et al. [43] | 46/M | Tibia (skeletal)† | Conventional/3 | S | NS | 96 | Lung | 96 | 54 | -/-/- | 98/2 | Dead |
| Kim et al. [44] | 37/F | Heart (soft tissue) | Conventional/NS | S+CT+RT | None | None | None | 20 | 39 | S/NS/NS | NS/NS | NS |
| Ozaki et al. [4] | 52/F | Femur (skeletal) | Conventional/3 | S+CT | NS | 4 | Lung | 4 | 52 | -/NS/NS | 14/10 | Dead |
| Rushing et al. [45] | 48/F | Caudal neuraxis (soft tissue) | Mesenchymal/3 | S (GTR)+RT | 24 | NS | Lung | NS | NS | NS/NS/NS | 84/NS | Dead |
| Sans et al. [46] | 22/M | Foot (skeletal) | Mesenchymal/3 | None | NS | 0 | Lung | 0 | 22 | -/-/- | 0.625/0.625 | Dead |
| Wroński et al. [47] | 30/F | Hand (skeletal) | NS/NS | NS | NS | NS | Lung | 35 | 33 | S/NS/NS | 39/4 | Dead |
| Wroński et al. [47] | 27/F | Craniofacial (skeletal) | NS/NS | NS | 1.25 | NS | No lung | 11 | 27 | S/NS/NS | 12.75/1.75 | Dead |
| Konishi et al. [48] | 28/F | Knee (skeletal) | Periosteal/NS | S+CT | 60 | 72 | Lung, ovary, spleen, stomach | 72 | 34 | S/-/- | 72/0 | Alive |
| Moran et al. [49] | 62/F | Larynx (soft tissue) | Conventional/NS | S (en bloc) | 12 | 15 | Lung, hypopharynx | 15 | 63 | -/-/- | NS/NS | Dead |
| Jallu et al. [50] | 45/F | Femur (skeletal) | Conventional/2 | S (wide) | None | None | None | 8 | 45 | GTR/-/- | NS/0.25 | Alive |
| Salminen et al. [51] | 68/M | Trachea (soft tissue)* | Conventional/NS | S (local) | NS | 12 | Rib | 36 | 71 | NS/NS/NS | 38/2 | Dead |
| Bertoni et al. [52] | 22/F | Tibia (skeletal)* | Dedifferentiated/3 | S (with margins) | 7 | NS | Lung, pelvis | NS | 22 | NS/NS/NS | 12/NS | Dead |
| Perry et al. [53], Bertoni et al. [54] | 51/F | Knee Cartilage (soft tissue)* | Conventional/2 | S (amputation)+CT | 15 | 15 | Lung | >15 | 53 | NS/NS/NS | 20/NS | Dead |
| Liu et al. [55] | 13/F | Femur (skeletal)† | NS/NS | S (amputation)+RT+CT | NS | NS | NS | NS | NS | NS/NS/NS | 12/NS | Dead |
| Liu et al. [55] | 64/F | Femur (skeletal)† | Dedifferentiated/3 | S (en bloc) | NS | NS | NS | NS | NS | NS/NS/NS | 2/NS | Dead |
| Reif and Graf [56] | 3/M | Caudal neuraxis (soft tissue) | Mesenchymal/3 | S (GTR)+CT+RT | 3 | None | None | 14 | 4 | STR/-/- | 15/1 | Dead |
| Johnson et al. [57] | 47/M | Pelvis | Dedifferentiated/3 | S (wide)+CT | NS | 5 | Lung, liver, adrenal, lymph nodes, bone marrow | 5 | 47 | NS/NS/NS | 6/1 | Dead |
| ohnson et al. [57] | 65/F | Pelvis (skeletal) | Dedifferentiated/3 | S (wide) | 3 | 3 | Liver, lung | 4 | 65 | NS/NS/NS | 4.5/0.5 | Dead |
| Johnson et al. [57] | 62/F | Femur (skeletal) | Dedifferentiated/3 | CT+RT | NS | 4 | Lung | 4 | 62 | NS/NS/NS | 6/2 | Dead |
| Sherr et al. [58] | 49/F | Pelvis (skeletal) | NS/NS | S (en bloc) | None | 72 | Lung, subcutaneous, oral cavity | 77 | 57 | S/-/- | 81/4 | Dead |
| Templeton et al. [59] | 20/M | Mandible (skeletal) | Conventional/NS | S | NS | 36 | Lung | 144 | 32 | S/NS/NS | NS/NS | NS |
| Pialat et al. [60] | 25/F | Arm (soft tissue) | Mesenchymal/3 | NS | None | NS, after brain | Lung, bone, skin, muscle | 24 | 27 | NS/NS/NS | 47/23 | Dead |
| Unni et al. [61] | NS/NS | Femur (skeletal) | Clear cell/1 | S | 84 | NS | Humerus, skull, lung, pelvis | 144 | NS | NS/NS/NS | 156/12 | Dead |
| Gercovich et al. [62] | 54/M | Pelvis (skeletal) | NS/NS | S (en bloc) | None | 43 | Lung, subcutaneous | 52 | 58 | -/-/- | 52.5/0.5 | Dead |
| Talerman [63] | 15/M | Tibia (skeletal) | Conventional/NS | S (amputation) | None | 228 | Kidney | 228 | 34 | -/-/- | 228/0 | Dead |
| Cowan [64] | 28/M | Tibia (skeletal)† | NS/NS | S (amputation) | NS | NS | NS | 30 | 30 | -/NS/NS | 30/0 | Dead |
*Secondary, nonsyndromic chondrosarcoma; †Secondary, syndromic chondrosarcoma. CNS, central nervous system; Tx, treatment; S, surgery; CT, chemotherapy; GTR, gross total resection; GK, gamma knife; STR, subtotal resection; RT, radiation; NS, not specified; -, none; WBRT, whole brain radiation therapy; BMT, bone marrow transplant
Table 3
Factors associated with time to development of brain metastases among patients with extracalvarial chondrosarcomas
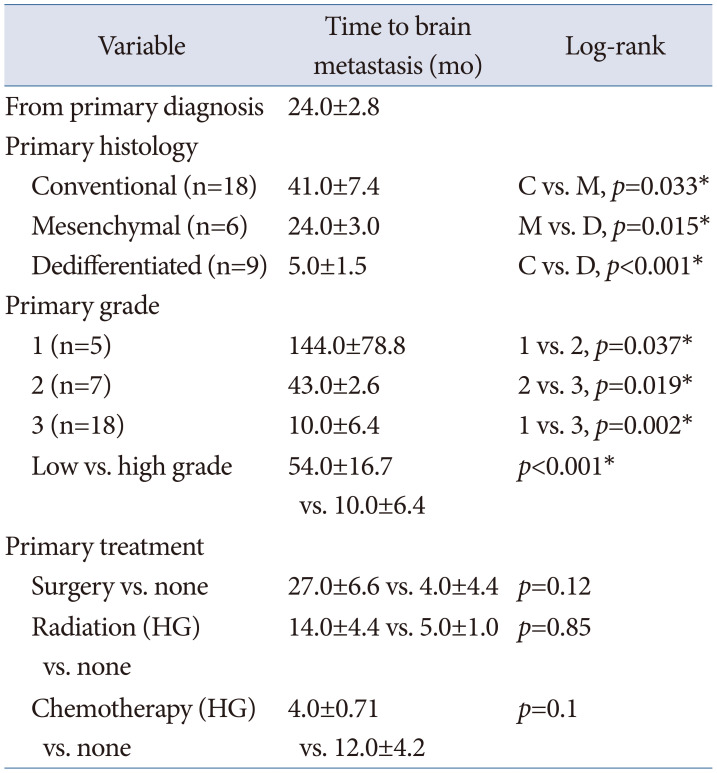
Table 4
Factors associated with overall survival following brain metastases among patients with extracalvarial chondrosarcomas




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download