This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Surgical intervention for brain tumor patients aged 80 to 89 years is controversial, as the comorbidities and physiology associated with aging are often thought to increase surgical risks. Surgical outcomes, however, are not well characterized for octogenarians. This review therefore assessed the outcomes and mortality risk associated with tumor removal in octogenarians at our academic institution.
Methods
Retrospective review of patients aged 80 to 89 who underwent craniotomy for tumor resection (CTR) at our institution between 2004–2021 and who were diagnosed with meningioma, glioblastoma, or metastatic disease. Primary outcome was 30-day mortality.
Results
Sixty-one CTRs were included in analysis. Median age was 83 (interquartile range 81–85) years, and the most common preoperative comorbidity was hypertension (n=44). Most patients (n=35) had a preoperative modified Rankin Scale (mRS) score between 0–2. Seventeen (27.9%) patients experienced postoperative complications (i.e., urinary tract infection, deep venous thrombosis, etc.), and 26.2% (n=16) experienced new-onset neurologic deficits postoperatively (i.e., aphasia, motor deficits, etc.). Upon discharge, most patients (n=43) had an mRS score of 3–4. Within 30 days of surgery, 14.8% (n=9) of patients were readmitted to the hospital and 8.2% (n=5) of patients died: 2 with meningioma, 1 with glioblastoma, and 2 with metastatic disease. The most common cause of death was intracranial hemorrhage (n=3). Three-month mortality was 23.0% (n=14). Mean survival after surgery was 33 months for meningioma patients, 6.9 months for glioblastoma patients, and 15 months for patients with metastatic lesions.
Conclusion
Our review found a 30-day mortality rate of 8.2% across all tumor types, and mean survival was similar to that previously reported for patients across all age groups. Surgical intervention for octogenarian tumor patients is therefore feasible, safe, and likely worthwhile for extending and improving lives.
Go to :

Keywords: Brain neoplasms, Craniotomy, Mortality, Octogenarians
INTRODUCTION
The incidence of both benign and malignant brain tumors increases with age [
123]; however, advanced patient age can result in hesitancy to surgically remove such tumors because of the potential comorbidities associated with aging that may increase surgical risks [
1]. Older age, usually considered 65 years or older, has been previously associated with greater risk of postoperative complications, prolonged hospital stay, and mortality within 30 days of surgery [
456]. On the other hand, many studies have identified frailty, rather than age, as the most important predictor of patient outcome following craniotomy for tumor resection (CTR) [
1789], suggesting the necessity to evaluate geriatric patients individually before ruling out CTR as a treatment option. Furthermore, outcomes for the sub-cohort of much older patients, particularly those >80 years of age, are not well-characterized, necessitating further examination of the efficacy and utility of CTR in this population.
To better comprehend the value of surgery in tumor management for octogenarians, we conducted a retrospective analysis of CTR patients aged 80–89 years, who were operated at our institution, examining surgical outcomes and mortality. Various patient factors were also examined to determine if certain comorbidities predict poorer surgical outcomes.
Go to :

METHODS
This retrospective review was approved by our Institutional Review Board (IRB, Study Number: 2021P000600) and was deemed exempt from patient consent by the IRB. Billing records identified all patients aged 80–89 at the time of surgery who underwent CTR or open biopsy between 2004 and 2021 at our academic medical center.
Patients with less than 3 months of follow-up were excluded from our analysis unless they died within 3 months of surgery, in which case they were included. Our study included only patients diagnosed with meningioma, glioblastoma, or metastatic tumors, based on their final pathology report.
All patient data, including pre-, intra-, and postoperative characteristics, were gleaned from the electronic medical record. Largest tumor diameter was determined from preoperative imaging reports. The degree of pre- and postoperative neurologic and functional disability was determined using the modified Rankin Scale (mRS) as the Karnofsky Performance Scores (KPS) were not available for many patients. Patients were grouped as mRS 0–2, 3–4, or 5–6 depending on functional status. Postoperative neurologic deficits and complications were defined as those that occurred during the inpatient period following surgery. Patients were grouped by diagnosis (meningioma, glioblastoma, or metastatic) for analysis and the primary outcome measure was 30-day mortality. Three-month mortality was also considered, and Kaplan-Meier (KM) estimator survival analysis was employed to evaluate overall patient survival. Comparative KM curves were developed to assess the effect of tumor type on patient survival over time. The log-rank test was used for assessing statistically significant differences in KM curve distributions. All patients were included in the survival analysis, and those who were lost to follow-up more than 3 months after surgery were censored at the time of last follow-up. A p-value of <0.05 was considered to be significant. All statistical analyses were performed using the Stata software (StataCorp, College Station, TX, USA).
Go to :

RESULTS
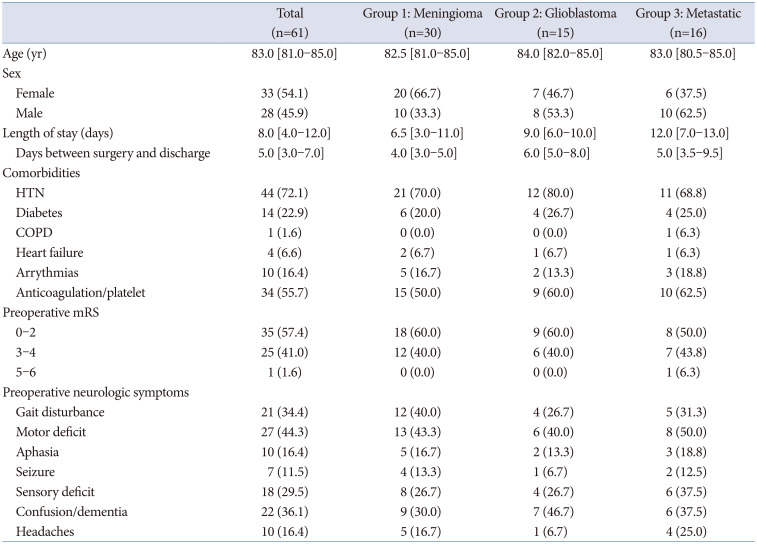
One hundred six billing records were identified, but 4 patients had 2 billing records associated with the same surgery (because 2 attending neurosurgeons were involved in their case), resulting in 102 unique craniotomies/biopsies. After excluding those without adequate follow-up (3 months) and anyone with a diagnosis other than glioblastoma, meningioma, or metastatic disease, 61 craniotomies (60 for tumor resection, 1 for open biopsy), performed on 60 unique patients, were included in this study. The median age at the time of surgery was 83 (interquartile range [IQR] 81–85) years. Twenty-nine patients (30 surgeries) were diagnosed with meningioma, 15 with glioblastoma, and 16 with metastatic disease (
Table 1).
Table 1
Patient demographics and preoperative data between the different tumor groups
|
Total (n=61) |
Group 1: Meningioma (n=30) |
Group 2: Glioblastoma (n=15) |
Group 3: Metastatic (n=16) |
|
Age (yr) |
83.0 [81.0–85.0] |
82.5 [81.0–85.0] |
84.0 [82.0–85.0] |
83.0 [80.5–85.0] |
|
Sex |
|
Female |
33 (54.1) |
20 (66.7) |
7 (46.7) |
6 (37.5) |
|
Male |
28 (45.9) |
10 (33.3) |
8 (53.3) |
10 (62.5) |
|
Length of stay (days) |
8.0 [4.0–12.0] |
6.5 [3.0–11.0] |
9.0 [6.0–10.0] |
12.0 [7.0–13.0] |
|
Days between surgery and discharge |
5.0 [3.0–7.0] |
4.0 [3.0–5.0] |
6.0 [5.0–8.0] |
5.0 [3.5–9.5] |
|
Comorbidities |
|
HTN |
44 (72.1) |
21 (70.0) |
12 (80.0) |
11 (68.8) |
|
Diabetes |
14 (22.9) |
6 (20.0) |
4 (26.7) |
4 (25.0) |
|
COPD |
1 (1.6) |
0 (0.0) |
0 (0.0) |
1 (6.3) |
|
Heart failure |
4 (6.6) |
2 (6.7) |
1 (6.7) |
1 (6.3) |
|
Arrythmias |
10 (16.4) |
5 (16.7) |
2 (13.3) |
3 (18.8) |
|
Anticoagulation/platelet |
34 (55.7) |
15 (50.0) |
9 (60.0) |
10 (62.5) |
|
Preoperative mRS |
|
0–2 |
35 (57.4) |
18 (60.0) |
9 (60.0) |
8 (50.0) |
|
3–4 |
25 (41.0) |
12 (40.0) |
6 (40.0) |
7 (43.8) |
|
5–6 |
1 (1.6) |
0 (0.0) |
0 (0.0) |
1 (6.3) |
|
Preoperative neurologic symptoms |
|
Gait disturbance |
21 (34.4) |
12 (40.0) |
4 (26.7) |
5 (31.3) |
|
Motor deficit |
27 (44.3) |
13 (43.3) |
6 (40.0) |
8 (50.0) |
|
Aphasia |
10 (16.4) |
5 (16.7) |
2 (13.3) |
3 (18.8) |
|
Seizure |
7 (11.5) |
4 (13.3) |
1 (6.7) |
2 (12.5) |
|
Sensory deficit |
18 (29.5) |
8 (26.7) |
4 (26.7) |
6 (37.5) |
|
Confusion/dementia |
22 (36.1) |
9 (30.0) |
7 (46.7) |
6 (37.5) |
|
Headaches |
10 (16.4) |
5 (16.7) |
1 (6.7) |
4 (25.0) |

Meningioma
The median age at the time of surgery for patients diagnosed with meningioma was 82.5 (IQR 81–85) years (n=30). Twenty-one patients (70%) had hypertension, and 15 (50%) were taking antiplatelet or anticoagulant medications preoperatively. Eighteen patients (60%) had preoperative mRS scores between 0–2, while 40% (n=12) had mRS scores of 3–4. The most common preoperative neurologic symptoms were gait disturbance (40%, n=12) and motor deficits (43.3%, n=13) (
Table 1).
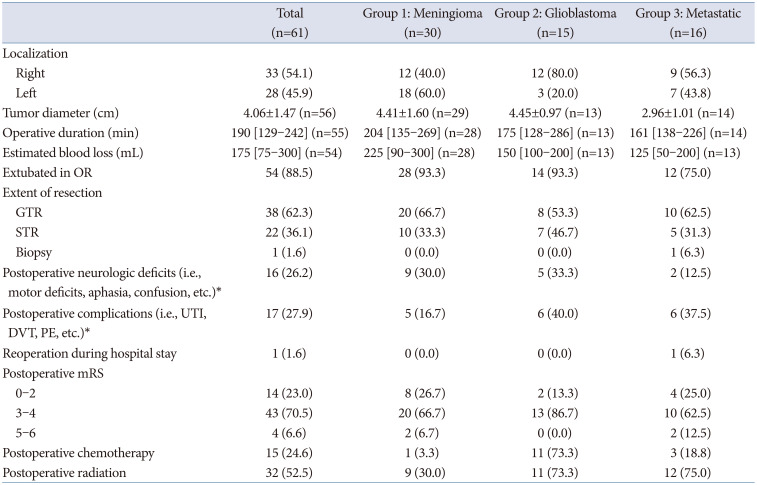
Eighteen meningiomas (60%) were removed from the left hemisphere, and average tumor size (defined as the greatest tumor diameter) was 4.41±1.60 cm. Gross total resection (GTR) was achieved for 66.7% (n=20) of patients, while 33.3% (n=10) of patients experienced subtotal resection (STR) (
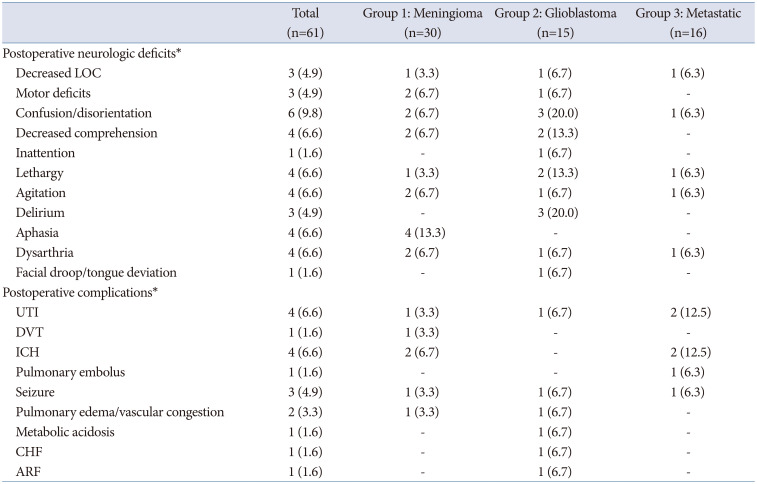
Table 2). Postoperatively, 30% (n=9) of patients had new-onset neurologic deficits, including motor deficits, confusion, and aphasia. Five patients (16.7%) experienced postoperative complications, including urinary tract infection (UTI; n=1), intracranial hemorrhage (ICH, n=2), deep venous thrombosis (DVT, n=1), and seizure (n=1). Postoperative complications and neurologic deficits are summarized in
Table 3. Upon discharge, 66.7% (n=20) of patients had mRS scores of 3–4, as 10 patients worsened from preoperative mRS of 0–2 to postoperative mRS of 3–4, and 2 patients worsened from preoperative mRS of 3–4 to postoperative mRS of 5–6 (
Table 2).
Table 2
Surgical details and postoperative outcomes between the different tumor groups
|
Total (n=61) |
Group 1: Meningioma (n=30) |
Group 2: Glioblastoma (n=15) |
Group 3: Metastatic (n=16) |
|
Localization |
|
Right |
33 (54.1) |
12 (40.0) |
12 (80.0) |
9 (56.3) |
|
Left |
28 (45.9) |
18 (60.0) |
3 (20.0) |
7 (43.8) |
|
Tumor diameter (cm) |
4.06±1.47 (n=56) |
4.41±1.60 (n=29) |
4.45±0.97 (n=13) |
2.96±1.01 (n=14) |
|
Operative duration (min) |
190 [129–242] (n=55) |
204 [135–269] (n=28) |
175 [128–286] (n=13) |
161 [138–226] (n=14) |
|
Estimated blood loss (mL) |
175 [75–300] (n=54) |
225 [90–300] (n=28) |
150 [100–200] (n=13) |
125 [50–200] (n=13) |
|
Extubated in OR |
54 (88.5) |
28 (93.3) |
14 (93.3) |
12 (75.0) |
|
Extent of resection |
|
GTR |
38 (62.3) |
20 (66.7) |
8 (53.3) |
10 (62.5) |
|
STR |
22 (36.1) |
10 (33.3) |
7 (46.7) |
5 (31.3) |
|
Biopsy |
1 (1.6) |
0 (0.0) |
0 (0.0) |
1 (6.3) |
|
Postoperative neurologic deficits (i.e., motor deficits, aphasia, confusion, etc.)*
|
16 (26.2) |
9 (30.0) |
5 (33.3) |
2 (12.5) |
|
Postoperative complications (i.e., UTI, DVT, PE, etc.)*
|
17 (27.9) |
5 (16.7) |
6 (40.0) |
6 (37.5) |
|
Reoperation during hospital stay |
1 (1.6) |
0 (0.0) |
0 (0.0) |
1 (6.3) |
|
Postoperative mRS |
|
0–2 |
14 (23.0) |
8 (26.7) |
2 (13.3) |
4 (25.0) |
|
3–4 |
43 (70.5) |
20 (66.7) |
13 (86.7) |
10 (62.5) |
|
5–6 |
4 (6.6) |
2 (6.7) |
0 (0.0) |
2 (12.5) |
|
Postoperative chemotherapy |
15 (24.6) |
1 (3.3) |
11 (73.3) |
3 (18.8) |
|
Postoperative radiation |
32 (52.5) |
9 (30.0) |
11 (73.3) |
12 (75.0) |

Table 3
Postoperative neurologic deficits and complications
|
Total (n=61) |
Group 1: Meningioma (n=30) |
Group 2: Glioblastoma (n=15) |
Group 3: Metastatic (n=16) |
|
Postoperative neurologic deficits*
|
|
Decreased LOC |
3 (4.9) |
1 (3.3) |
1 (6.7) |
1 (6.3) |
|
Motor deficits |
3 (4.9) |
2 (6.7) |
1 (6.7) |
- |
|
Confusion/disorientation |
6 (9.8) |
2 (6.7) |
3 (20.0) |
1 (6.3) |
|
Decreased comprehension |
4 (6.6) |
2 (6.7) |
2 (13.3) |
- |
|
Inattention |
1 (1.6) |
- |
1 (6.7) |
- |
|
Lethargy |
4 (6.6) |
1 (3.3) |
2 (13.3) |
1 (6.3) |
|
Agitation |
4 (6.6) |
2 (6.7) |
1 (6.7) |
1 (6.3) |
|
Delirium |
3 (4.9) |
- |
3 (20.0) |
- |
|
Aphasia |
4 (6.6) |
4 (13.3) |
- |
- |
|
Dysarthria |
4 (6.6) |
2 (6.7) |
1 (6.7) |
1 (6.3) |
|
Facial droop/tongue deviation |
1 (1.6) |
- |
1 (6.7) |
- |
|
Postoperative complications*
|
|
UTI |
4 (6.6) |
1 (3.3) |
1 (6.7) |
2 (12.5) |
|
DVT |
1 (1.6) |
1 (3.3) |
- |
- |
|
ICH |
4 (6.6) |
2 (6.7) |
- |
2 (12.5) |
|
Pulmonary embolus |
1 (1.6) |
- |
- |
1 (6.3) |
|
Seizure |
3 (4.9) |
1 (3.3) |
1 (6.7) |
1 (6.3) |
|
Pulmonary edema/vascular congestion |
2 (3.3) |
1 (3.3) |
1 (6.7) |
- |
|
Metabolic acidosis |
1 (1.6) |
- |
1 (6.7) |
- |
|
CHF |
1 (1.6) |
- |
1 (6.7) |
- |
|
ARF |
1 (1.6) |
- |
1 (6.7) |
- |

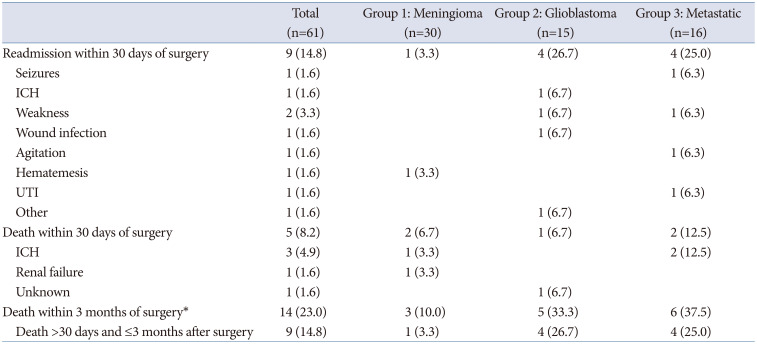
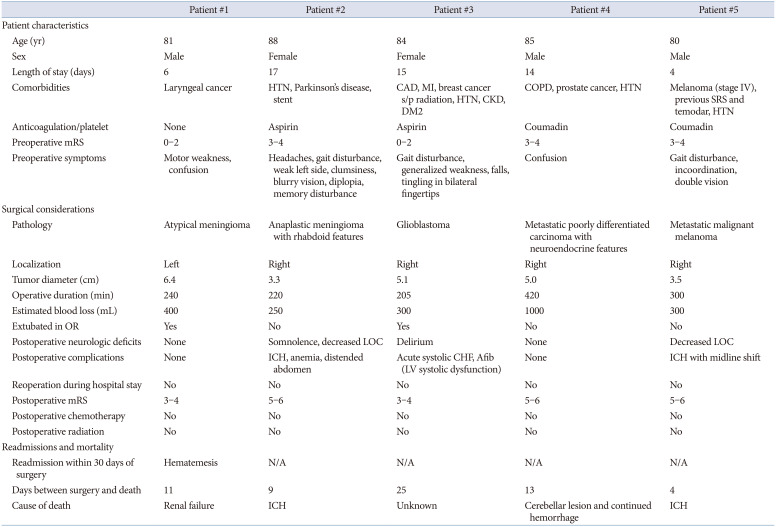
The 30-day mortality rate for patients with meningioma was 6.7% (n=2). One patient, who had a preoperative mRS score between 0–2, was readmitted within 30 days after surgery for hematemesis, and ultimately died of renal failure 11 days after surgery. The other patient, who had a past medical history of hypertension, was regularly taking aspirin preoperatively, and who had a preoperative mRS score of 3–4, died of an intracranial hemorrhage 9 days after surgery. One additional patient (3.3%) died within 3 months following surgery (
Tables 4 and
5).
Table 4
Readmissions and mortality
|
Total (n=61) |
Group 1: Meningioma (n=30) |
Group 2: Glioblastoma (n=15) |
Group 3: Metastatic (n=16) |
|
Readmission within 30 days of surgery |
9 (14.8) |
1 (3.3) |
4 (26.7) |
4 (25.0) |
|
Seizures |
1 (1.6) |
|
|
1 (6.3) |
|
ICH |
1 (1.6) |
|
1 (6.7) |
|
|
Weakness |
2 (3.3) |
|
1 (6.7) |
1 (6.3) |
|
Wound infection |
1 (1.6) |
|
1 (6.7) |
|
|
Agitation |
1 (1.6) |
|
|
1 (6.3) |
|
Hematemesis |
1 (1.6) |
1 (3.3) |
|
|
|
UTI |
1 (1.6) |
|
|
1 (6.3) |
|
Other |
1 (1.6) |
|
1 (6.7) |
|
|
Death within 30 days of surgery |
5 (8.2) |
2 (6.7) |
1 (6.7) |
2 (12.5) |
|
ICH |
3 (4.9) |
1 (3.3) |
|
2 (12.5) |
|
Renal failure |
1 (1.6) |
1 (3.3) |
|
|
|
Unknown |
1 (1.6) |
|
1 (6.7) |
|
|
Death within 3 months of surgery*
|
14 (23.0) |
3 (10.0) |
5 (33.3) |
6 (37.5) |
|
Death >30 days and ≤3 months after surgery |
9 (14.8) |
1 (3.3) |
4 (26.7) |
4 (25.0) |

Table 5
Patient characteristics related to thirty-day mortality
|
Patient #1 |
Patient #2 |
Patient #3 |
Patient #4 |
Patient #5 |
|
Patient characteristics |
|
Age (yr) |
81 |
88 |
84 |
85 |
80 |
|
Sex |
Male |
Female |
Female |
Male |
Male |
|
Length of stay (days) |
6 |
17 |
15 |
14 |
4 |
|
Comorbidities |
Laryngeal cancer |
HTN, Parkinson’s disease, stent |
CAD, MI, breast cancer s/p radiation, HTN, CKD, DM2 |
COPD, prostate cancer, HTN |
Melanoma (stage IV), previous SRS and temodar, HTN |
|
Anticoagulation/platelet |
None |
Aspirin |
Aspirin |
Coumadin |
Coumadin |
|
Preoperative mRS |
0–2 |
3–4 |
0–2 |
3–4 |
3–4 |
|
Preoperative symptoms |
Motor weakness, confusion |
Headaches, gait disturbance, weak left side, clumsiness, blurry vision, diplopia, memory disturbance |
Gait disturbance, generalized weakness, falls, tingling in bilateral fingertips |
Confusion |
Gait disturbance, incoordination, double vision |
|
Surgical considerations |
|
Pathology |
Atypical meningioma |
Anaplastic meningioma with rhabdoid features |
Glioblastoma |
Metastatic poorly differentiated carcinoma with neuroendocrine features |
Metastatic malignant melanoma |
|
Localization |
Left |
Right |
Right |
Right |
Right |
|
Tumor diameter (cm) |
6.4 |
3.3 |
5.1 |
5.0 |
3.5 |
|
Operative duration (min) |
240 |
220 |
205 |
420 |
300 |
|
Estimated blood loss (mL) |
400 |
250 |
300 |
1000 |
300 |
|
Extubated in OR |
Yes |
No |
Yes |
No |
No |
|
Postoperative neurologic deficits |
None |
Somnolence, decreased LOC |
Delirium |
None |
Decreased LOC |
|
Postoperative complications |
None |
ICH, anemia, distended abdomen |
Acute systolic CHF, Afib (LV systolic dysfunction) |
None |
ICH with midline shift |
|
Reoperation during hospital stay |
No |
No |
No |
No |
No |
|
Postoperative mRS |
3–4 |
5–6 |
3–4 |
5–6 |
5–6 |
|
Postoperative chemotherapy |
No |
No |
No |
No |
No |
|
Postoperative radiation |
No |
No |
No |
No |
No |
|
Readmissions and mortality |
|
Readmission within 30 days of surgery |
Hematemesis |
N/A |
N/A |
N/A |
N/A |
|
Days between surgery and death |
11 |
9 |
25 |
13 |
4 |
|
Cause of death |
Renal failure |
ICH |
Unknown |
Cerebellar lesion and continued hemorrhage |
ICH |

Glioblastoma
The median age at surgery for patients with glioblastoma was 84 (IQR 82–85) years (n=15). Twelve patients (80%) had hypertension, and 9 patients (60%) were using anticoagulant and/or antiplatelet medications preoperatively. Preoperative neurologic symptoms included gait disturbance (26.7%, n=4), motor deficits (40.0%, n=6), sensory deficits (26.7%, n=4), and confusion/dementia (46.7%, n=7). Nine patients (60%) had mRS scores between 0–2, and 6 patients (40%) had mRS scores of 3–4 (
Table 1).
Twelve tumors (80%) were in the right hemisphere; the average tumor size was 4.45±0.97 cm. A GTR was achieved in 53.3% (n=8) of patients, and an STR was achieved in 46.7% (n=7) (
Table 2). One third (n=5) of patients experienced new-onset neurologic deficits postoperatively, the most common being confusion/disorientation (n=3) and delirium (n=3). Six patients (40%) experienced postoperative complications, including UTI (n=1), seizure (n=1), pulmonary edema/vascular congestion (n=1), metabolic acidosis (n=1), congestive heart failure (n=1), and acute renal failure (n=1) (
Table 3). Upon discharge, 86.7% (n=13) of patients had mRS scores of 3–4, as 7 patients worsened from a preoperative mRS of 0–2 to a postoperative mRS of 3–4. Eleven patients (73.3%) underwent chemotherapy and radiaton following surgery (
Table 2).
Four (26.7%) glioblastoma patients were readmitted within 30 days of surgery: 1 for ICH, 1 for generalized weakness, 1 for a craniotomy wound infection, and 1 for generalized fatigue and infection at their port-a-cath site. The 30-day mortality rate was 6.7% (n=1), as 1 patient, who was not readmitted, died 25 days after surgery for an unknown reason. This patient had a preoperative mRS score between 0–2; a past medical history of hypertension, diabetes, coronary artery disease, and myocardial infarction; and was regularly taking aspirin preoperatively. Four additional patients (26.7%) died within 3 months after surgery (
Tables 4 and
5).
Metastatic disease
The median age at surgery for the 16 patients diagnosed with metastatic disease was 83 (IQR 80.5–85) years. Eleven (69%) of patients had hypertension, and 10 (62.5%) were using anticoagulation and/or antiplatelet medications before surgery. Preoperative neurologic symptoms included gait disturbance (31.3%, n=5), motor deficits (50.0%, n=8), sensory deficits (37.5%, n=6), and confusion/dementia (37.5%, n=6). Eight patients (50.0%) had preoperative mRS scores between 0–2, and 43.8% (n=7) of patients had mRS scores of 3–4. One patient had an mRS score of 5 (
Table 1).
Nine tumors (56.3%) were in the right hemisphere, and the mean tumor size was 2.96±1.01 cm. A GTR was achieved in 62.5% (n=10), and an STR in 31.3% (n=5). One patient (6.3%) underwent an open biopsy instead of full resection (
Table 2). Two patients (12.5%) experienced new-onset, postoperative neurologic deficits: one showed a decreased level of consciousness, and the other had confusion, dysarthria, lethargy, and agitation. Six patients (37.5%) experienced postoperative complications, including UTI (n=2), ICH (n=2), pulmonary embolus (PE) (n=1), and seizure (n=1) (
Table 3). One patient who experienced ICH required an additional operation during the same hospital stay as their initial craniotomy. Upon discharge, 62.5% (n=10) of patients had an mRS score of 3–4, as 4 patients worsened from preoperative mRS of 0–2 to postoperative mRS of 3–4, 2 patients worsened from preoperative mRS of 3–4 to postoperative mRS of 5–6, and 1 patient improved from preoperative mRS of 5–6 to postoperative mRS of 3–4 (
Table 2).
Four patients were readmitted within 30 days of surgery: one for seizures, one for generalized weakness, one for agitation, and one for a UTI. The 30-day mortality rate for patients with metastatic disease was 12.5% (n=2), as 2 patients, both of whom had a preoperative mRS of 3–4, a history of hypertension, and were on warfarin (Coumadin) preoperatively, died due to ICH. Four additional patients (25%) died within 3 months of surgery (
Tables 4 and
5).
Overall 30-day and 3-month mortality
Across all tumor types, the 30-day mortality rate was 8.2% (n=5). An additional 14.8% (n=9) of patients died between 30 days and 3 months of surgery, yielding a 90-day mortality rate of 23.0%.
KM survival analysis
KM survival analysis included all patients in the study (30 meningioma, 15 glioblastoma, and 16 metastatic disease). The mean survival was 33 months for the meningioma group, 6.9 months for the glioblastoma group, and 15 months for the metastatic lesion group (
Fig. 1). Differences in mean survival between tumor types were statistically significant (
p<0.001). The KM survival curve is shown in
Fig. 1.
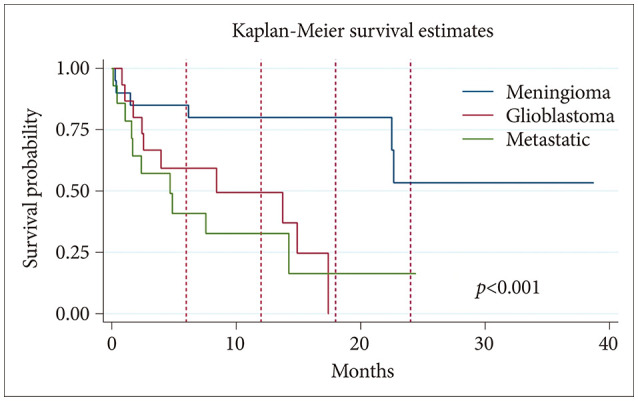 | Fig. 1Kaplan-Meier survival analysis curve for patients with meningioma, glioblastoma, or metastatic disease. Kaplan-Meier survival analysis was performed to evaluate overall longevity following surgery for octogenarian patients who underwent craniotomy for tumor resection. A total of 30 meningioma, 15 glioblastoma, and 16 metastatic lesion patients were analyzed. Mean survival was 33 months for the meningioma group, 6.9 months for the glioblastoma group, and 15 months postsurgery for the metastatic lesion group. Red dash lines indicate follow-up at 6, 12, 18, and 24 months. Log-rank test was significant with p<0.001.
|
Go to :

DISCUSSION
Through analyzing surgical outcomes and both 30- and 90-day mortality of octogenarian CTR patients at our hospital, this study sought to assess the feasibility and safety of CTR for brain tumor patients aged 80 to 89 years. The 30-day mortality rate across all tumor types at our center was 8.2% (n=5), and the 90-day mortality rate was 23% (n=14). KM survival analysis demonstrated mean survival of 33 months for meningioma patients, 6.9 months for glioblastoma patients, and 15 months for patients with metastatic lesions in our cohort.
The decision to operate
Presently, there is little consensus regarding whether or not patients aged 80 years or older should undergo CTR. In one multicenter review of 1,281 meningioma patients who underwent CTR, elderly age (defined as ≥70 years) (n=258) was associated with a higher 30-day mortality rate (12.0%) compared to younger patients (4.6%), and elderly patients were more likely to have one or more complications (29.8%) compared to their younger counterparts (13.1%) [
5]. In another review of meningioma patients who underwent CTR, age greater than 80 years was an independent risk factor for complications, death within 30 days of surgery, and prolonged length of stay (>5 days) [
6].
In contrast, many other studies have found no association between elderly age and increased risk for complications or mortality following CTR suggesting that CTR is a safe procedure for geriatric patients. For elderly patients who do experience complications, ICH, seizure, DVT, PE, infection, and stroke are most common [
17810111213141516].
Our study demonstrated a 30-day mortality rate of 8.2% (n=5) across all tumor types, and though 27.9% of patients experienced a postoperative complication (i.e., UTI, DVT, PE, etc.), most complications did not lead to death and were resolved quickly. An additional 9 patients (4 with glioblastoma, 4 with metastatic lesions, and 1 with meningioma) died between 30 days and 3 months following surgery; however, this result was not unexpected given the aggressive nature of glioblastoma and metastatic tumors. We therefore argue that CTR for patients aged 80 to 89 years is overall feasible and relatively safe for most patients.
According to the Central Brain Tumor Registry of the United States (CBTRUS), median survival for patients with glioblastoma across all age groups is 8 months from the time of diagnosis [
3]. KM survival analysis demonstrated that mean survival in our glioblastoma cohort was 6.9 months from time of surgery. Given that we measured survival as time from surgery instead of time from diagnosis (which may occur before CTR through radiographic confirmation), CTR in our octogenarian cohort does not appear to reduce overall longevity for glioblastoma patients. For meningioma patients, per the CBTRUS, 10-year relative survival across all age groups for malignant meningioma is 67%; however, for patients 75 years or older, 10-year relative survival is reduced to 40%. Ten-year relative survival for non-malignant meningioma is 83% across all age groups, but it also is reduced as age increases [
3]. In our cohort, the mean survival for meningioma patients was 33 months, and at least 7 patients (23.3%) survived or are still alive more than 5 years postsurgery. While most meningiomas are not malignant, older age increases a patient’s likelihood of dying from other causes, making it difficult to compare overall survival in our meningioma patients to patients of younger age. Mean survival for patients with metastatic lesions was 15 months in our cohort, and it is likely that many patients with metastatic lesions succumb to comorbidities associated with their systemic disease rather than issues related to CTR. Our survival analysis, therefore, suggests the safety and efficacy of CTR in patients aged over 80–89 years.
Additionally, although mRS score was worse for several patients upon discharge compared to preoperative status, need for additional care immediately following surgery (i.e., rehabilitation and home services) is not abnormal for neurosurgical patients, and it is likely that many patients later improved in functional status. CTR for patients of very advanced age may therefore still be of value for increasing longevity and improving patients’ quality of life.
Risk factors for poor outcomes
Careful patient selection, as is true for most surgical procedures, is important for minimizing negative surgical outcomes. In the literature, most research argues that geriatric patients with decreased functional or neurologic status preoperatively (as measured by mRS, KPS, and/or American Society of Anesthesiologists [ASA] score) are at greater risk for postoperative complications or mortality [
1789]. For example, in one review of 58 patients aged 80 years or older who underwent CTR for glioblastoma, a lower KPS (≤60) was associated with lower overall survival [
9]. In a different review of 58 meningioma patients of age 70 or older, mortality was associated with KPS ≤60 and an ASA score of 3 [
1].
In our study, 2 of the patients who died within 30 days of surgery had preoperative mRS scores between 0–2, suggesting strong functional and neurologic status. The other 3 patients who died within 30 days had preoperative mRS scores of 3–4. Neurologic and functional status were therefore not associated with higher risk of mortality in our review. Preoperative symptoms also differed between all 5 patients who died within 30 days of surgery, making us unable to pinpoint any particular symptom that predicts worse surgical outcomes.
Besides neurologic and functional status, no other patient characteristics have been consistently associated with increased risk of mortality after CTR in elderly cohorts. Of the 5 patients who died within 30 days of surgery in our review, 4 (3 of whom died from hemorrhage) had a history of hypertension and were taking anticoagulants and/or antiplatelets preoperatively. Hypertension (present in 72.1% of all patients) and anticoagulants/platelets (used by 55.7% of all patients) were common in our cohort, however, while we cannot definitively demonstrate a relationship between such factors and mortality following CTR, it is possible that hypertension and anticoagulant/platelet use contributed to an increased risk of ICH and mortality.
The maximum tumor diameters of 3 of the 5 patients who died were slightly larger than average compared to others in the cohort. Additionally, the estimated blood loss of those patients who died was larger compared to the overall average for each tumor type, and 3 of the 5 patients were unable to be extubated in the operating room at the conclusion of surgery. It is possible that increased blood loss and prolonged intubation may have contributed to the poor outcomes in these patients (
Table 5).
While we were unable to pinpoint preoperative factors that differentiated the patients in our cohort who died within 30 days of surgery from their surviving counterparts, postoperatively, it is possible that factors such as blood loss and respiratory status may be indicative of patient outcomes. More research is needed to draw firm conclusions regarding these postoperative factors. Overall, the results of our study support the decision to take octogenarian patients to the operating room, though as is true for any age group, patients should be evaluated on a case-by-case basis to determine personal surgical risk.
Generalizability and limitations
Given that this review only included patients who underwent CTR at our academic institution with our surgeons, generalizing our results to all neurosurgical departments may not be feasible. That said, our review adds to literature from other institutions that suggests CTR as a safe and worthwhile procedure for octogenarian patients.
The biggest limitation of this review was possible selection bias, as our cohort represents only those patients that our surgeons deemed fit enough to undergo surgery. Consequently, there are likely many geriatric patients excluded from this study who would not be good candidates for CTR for various reasons. Careful patient selection probably has an important impact on surgical outcomes that we were unable to wholly assess in this review.
Quality of life is also essential to consider when deciding whether to operate on patients of advanced age. Although CTR may be feasible and safe for such patients, the level of care patients will need postoperatively, how much independence they will retain, and how surgery is predicted to affect their overall survival must play a role in the decision to operate. Our review did not assess long-term quality of life, necessitating further research.
Conclusions
CTR in patients aged 80 to 89 years can be controversial because of concerns that advanced age makes patients less physiologically and neurologically fit, thereby increasing the risks of postoperative morbidity and mortality. In line with previous research, this review of 61 CTRs for meningioma, glioblastoma, and metastatic tumors demonstrated that CTR is feasible and a relatively safe procedure for octogenarians. Although patients, including those younger than 80 years, should always be evaluated on a case-by-case basis for surgical risks, we believe that surgical interventions for octogenarian patients harboring brain tumors should be considered a realistic treatment option.
Go to :

Notes
Go to :

Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Czernicki T. Surgical management of intracranial meningiomas in the elderly: early and long-term outcomes. Clin Interv Aging. 2020; 15:2439–2451. PMID:
33408468.
2. Chen B, Chen C, Zhang Y, Xu J. Recent incidence trend of elderly patients with glioblastoma in the United States, 2000-2017. BMC Cancer. 2021; 21:54. PMID:
33430813.
3. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021; 23(12 Suppl 2):iii1–iii105. PMID:
34608945.
4. Garcia CM, Pertsch NJ, Leary OP, Rivera Perla KM, Tang O, Toms SA, et al. Early outcomes of supratentorial cranial surgery for tumor resection in older patients. J Clin Neurosci. 2021; 83:88–95. PMID:
33342625.
5. Patil CG, Veeravagu A, Lad SP, Boakye M. Craniotomy for resection of meningioma in the elderly: a multicentre, prospective analysis from the national surgical quality improvement program. J Neurol Neurosurg Psychiatry. 2010; 81:502–505. PMID:
19828483.
6. Steinberger J, Bronheim RS, Vempati P, Oermann EK, Ladner TR, Lee NJ, et al. Morbidity and mortality of meningioma resection increases in octogenarians. World Neurosurg. 2018; 109:e16–e23. PMID:
28919230.
7. Schär RT, Tashi S, Branca M, Söll N, Cipriani D, Schwarz C, et al. How safe are elective craniotomies in elderly patients in neurosurgery today? A prospective cohort study of 1452 consecutive cases. J Neurosurg. 2020; 134:1113–1121. PMID:
32330879.
8. Corniola MV, Lemée JM, Meling TR. Resection of meningiomas in octogenarians: a comparison with a younger geriatric population. Neurosurg Focus. 2020; 49:E18.
9. Abdullah KG, Ramayya A, Thawani JP, Macyszyn L, Martinez-Lage M, O’Rourke DM, et al. Factors associated with increased survival after surgical resection of glioblastoma in octogenarians. PLoS One. 2015; 10:e0127202. PMID:
25978638.
10. Ferroli P, Vetrano IG, Schiavolin S, Acerbi F, Zattra CM, Schiariti M, et al. Brain tumor resection in elderly patients: potential factors of postoperative worsening in a predictive outcome model. Cancers (Basel). 2021; 13:2320. PMID:
34065990.
11. Maldaner N, Sarnthein J, Bozinov O, Regli L, Neidert MC. Neurosurgery in octogenarians: a prospective study of perioperative morbidity, mortality, and complications in elderly patients. World Neurosurg. 2018; 110:e287–e295. PMID:
29113899.
12. Lopez-Rivera V, Dono A, Lewis CT, Chandra A, Abdelkhaleq R, Sheth SA, et al. Extent of resection and survival outcomes of geriatric patients with glioblastoma: is there benefit from aggressive surgery? Clin Neurol Neurosurg. 2021; 202:106474. PMID:
33454497.
13. Seicean A, Seicean S, Schiltz NK, Alan N, Jones PK, Neuhauser D, et al. Short-term outcomes of craniotomy for malignant brain tumors in the elderly. Cancer. 2013; 119:1058–1064. PMID:
23065678.
14. Eichberg DG, Di L, Shah AH, Luther E, Richardson AM, Sarkiss CA, et al. Brain tumor surgery is safe in octogenarians and nonagenarians: a single-surgeon 741 patient series. World Neurosurg. 2019; 132:e185–e192. PMID:
31505286.
15. Zoia C, Bongetta D, Guerrini F, Alicino C, Cattalani A, Bianchini S, et al. Outcome of elderly patients undergoing intracranial meningioma resection: a single-center experience. J Neurosurg Sci. 2021; 65:513–517. PMID:
29808631.
16. Ikawa F, Kinoshita Y, Takeda M, Saito T, Yamaguchi S, Yamasaki F, et al. Review of current evidence regarding surgery in elderly patients with meningioma. Neurol Med Chir (Tokyo). 2017; 57:521–533. PMID:
28819091.
Go to :











 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download