1. Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019; 11(8):762. PMID:
31430946.
2. Dorward DA, Russell CD, Um IH, Elshani M, Armstrong SD, Penrice-Randal R, et al. Tissue-specific immunopathology in fatal COVID-19. Am J Respir Crit Care Med. 2021; 203(2):192–201. PMID:
33217246.
3. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395(10229):1033–1034. PMID:
32192578.
4. McElvaney OJ, McEvoy NL, McElvaney OF, Carroll TP, Murphy MP, Dunlea DM, et al. Characterization of the inflammatory response to severe COVID-19 disease. Am J Respir Crit Care Med. 2020; 202(6):812–821. PMID:
32584597.
5. Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020; 56(6):2002808. PMID:
32943404.
6. Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P, Marjani M, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021; 897:173947. PMID:
33607104.
7. Papamanoli A, Yoo J, Grewal P, Predun W, Hotelling J, Jacob R, et al. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. Eur J Clin Invest. 2021; 51(2):e13458. PMID:
33219551.
8. López Zúñiga MÁ, Moreno-Moral A, Ocaña-Granados A, Padilla-Moreno FA, Castillo-Fernández AM, Guillamón-Fernández D, et al. High-dose corticosteroid pulse therapy increases the survival rate in COVID-19 patients at risk of hyper-inflammatory response. PLoS One. 2021; 16(1):e0243964. PMID:
33507958.
9. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020; 369:m1985. PMID:
32444460.
10. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020; 324(13):1330–1341. PMID:
32876694.
11. Kumar G, Patel D, Hererra M, Jefferies D, Sakhuja A, Meersman M, et al. Do high-dose corticosteroids improve outcomes in hospitalized COVID-19 patients? J Med Virol. 2022; 94(1):372–379. PMID:
34559436.
12. Swaminathan L, Kaatz S, Chubb H, Tae K, Ramesh MS, Fadel R, et al. Impact of early corticosteroids on preventing clinical deterioration in non-critically ill patients hospitalized with COVID-19: a multi-hospital cohort study. Infect Dis Ther. 2022; 11(2):887–898. PMID:
35267172.
13. Noguchi K, Latif M, Thangavelu K, Konietschke F, Gel YR, Brunner E. nparLD: Nonparametric analysis of longitudinal data in factorial experiments, R package version 2.1. Updated 2012. Accessed October 13, 2022.
https://cran.r-project.org/web/packages/nparLD
.
14. Wang J, Yang W, Chen P, Guo J, Liu R, Wen P, et al. The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: a systematic review and meta-analysis. PLoS One. 2021; 16(4):e0249481. PMID:
33882090.
15. Becker DE. Basic and clinical pharmacology of glucocorticosteroids. Anesth Prog. 2013; 60(1):25–31. PMID:
23506281.
16. Zhan Y, Shang J, Gu Y, Huang Q, Xie J. Efficacy of corticosteroid in patients with COVID-19: a multi-center retrospective study and meta-analysis. J Med Virol. 2021; 93(7):4292–4302. PMID:
33666250.
17. Tortajada C, Colomer E, Andreu-Ballester JC, Esparcia A, Oltra C, Flores J. Corticosteroids for COVID-19 patients requiring oxygen support? Yes, but not for everyone: effect of corticosteroids on mortality and intensive care unit admission in patients with COVID-19 according to patients’ oxygen requirements. J Med Virol. 2021; 93(3):1817–1823. PMID:
33107607.
18. Keller MJ, Kitsis EA, Arora S, Chen JT, Agarwal S, Ross MJ, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020; 15(8):489–493. PMID:
32804611.
19. RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021; 384(8):693–704. PMID:
32678530.
20. Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020; 8(3):267–276. PMID:
32043986.
21. Yang R, Xiong Y, Ke H, Chen T, Gao S. The role of methylprednisolone on preventing disease progression for hospitalized patients with severe COVID-19. Eur J Clin Invest. 2020; 50(11):e13412. PMID:
32954492.
22. Pinzón MA, Ortiz S, Holguín H, Betancur JF, Cardona Arango D, Laniado H, et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS One. 2021; 16(5):e0252057. PMID:
34033648.
23. Ranjbar K, Moghadami M, Mirahmadizadeh A, Fallahi MJ, Khaloo V, Shahriarirad R, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021; 21(1):337. PMID:
33838657.
24. Ko JJ, Wu C, Mehta N, Wald-Dickler N, Yang W, Qiao R. A comparison of methylprednisolone and dexamethasone in intensive care patients with COVID-19. J Intensive Care Med. 2021; 36(6):673–680. PMID:
33632000.
25. Jamil Z, Almajhdi FN, Khalid S, Asghar M, Ahmed J, Waheed Y. Comparison of low-versus high-dose steroids in the clinical outcome of hospitalized COVID-19 patients. Antibiotics (Basel). 2021; 10(12):1510. PMID:
34943722.
26. Monreal E, Sainz de la Maza S, Natera-Villalba E, Beltrán-Corbellini Á, Rodríguez-Jorge F, Fernández-Velasco JI, et al. High versus standard doses of corticosteroids in severe COVID-19: a retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2021; 40(4):761–769. PMID:
33083917.
27. Chen Y, Li L. Influence of corticosteroid dose on viral shedding duration in patients with COVID-19. Clin Infect Dis. 2021; 72(7):1298–1300. PMID:
32588884.
28. Bivona G, Agnello L, Ciaccio M. Biomarkers for prognosis and treatment response in COVID-19 patients. Ann Lab Med. 2021; 41(6):540–548. PMID:
34108281.
29. Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. 2020; 35(13):e142. PMID:
32242348.
30. Soliman OM, Moeen SM, Abbas YA, Kamel EZ. The impact of dexamethasone versus methylprednisolone upon neutrophil/lymphocyte ratio in COVID-19 patients admitted to ICU and its implication upon mortality. Egypt J Anaesth. 2022; 38(1):78–84.
31. Li S, Hu Z, Song X. High-dose but not low-dose corticosteroids potentially delay viral shedding of patients with COVID-19. Clin Infect Dis. 2021; 72(7):1297–1298. PMID:
32588877.
32. Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): a randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2021; 72(9):e373–e381. PMID:
32785710.
33. Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020; 324(13):1317–1329. PMID:
32876697.
34. van Paassen J, Vos JS, Hoekstra EM, Neumann KM, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020; 24(1):696. PMID:
33317589.
35. Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020; 324(13):1298–1306. PMID:
32876689.
36. Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020; 324(13):1307–1316. PMID:
32876695.
37. Ritter LA, Britton N, Heil EL, Teeter WA, Murthi SB, Chow JH, et al. The impact of corticosteroids on secondary infection and mortality in critically ill COVID-19 patients. J Intensive Care Med. 2021; 36(10):1201–1208. PMID:
34247526.
38. Oh SM, Ham SY, Suh HJ, Lee E, Park SW. Clinical characteristics of COVID-19: use of steroids in mostly unvaccinated COVID-19 patients before the omicron variant. J Korean Med Sci. 2022; 37(29):e228. PMID:
35880504.
39. Na YS, Baek AR, Baek MS, Kim WY, Kim JH, Lee BY, et al. Clinical outcomes of and risk factors for secondary infection in patients with severe COVID-19: a multicenter cohort study in South Korea. Korean J Intern Med. 2023; 38(1):68–79. PMID:
36420564.
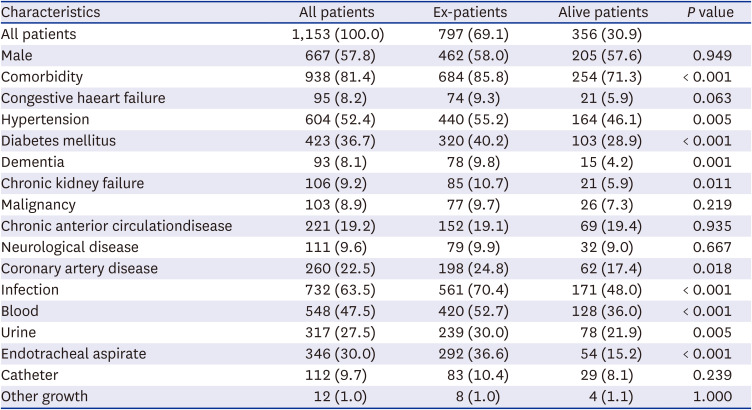
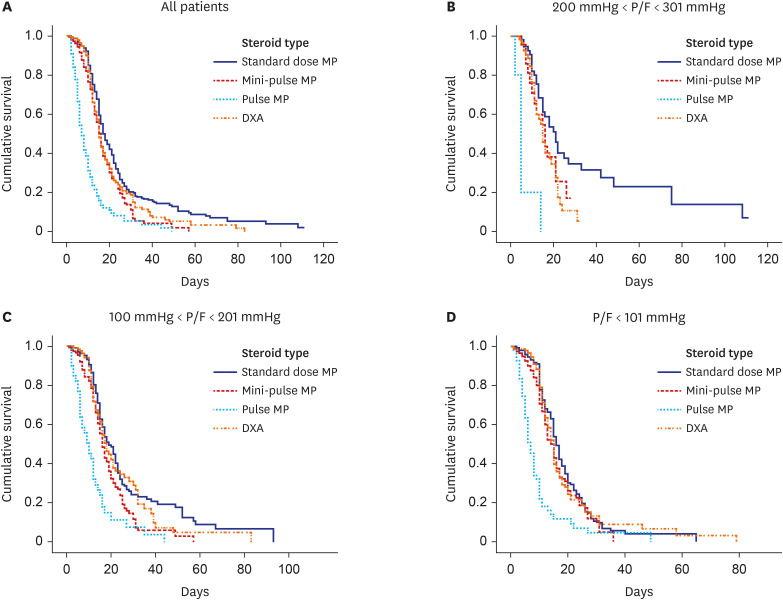
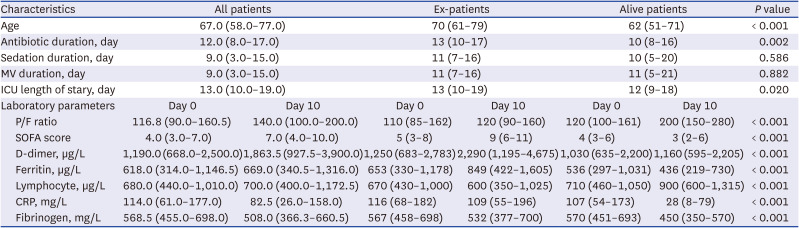
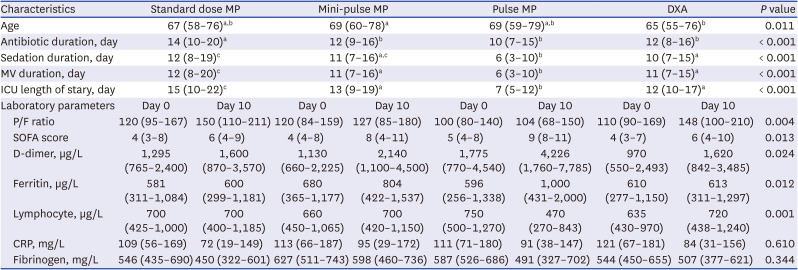




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download