CASE DESCRIPTION
A 27-year-old woman presented to the emergency room (ER) after being rescued from the Halloween crowd crush in Itaewon. Due to overcrowding in a narrow, sloped alleyway, she fell over and was trampled by people for 1.5 hours before being rescued.
Immediately after visiting the ER, her mental status was drowsy. She complained of severe pain in her lower back and the entireties of both legs with weakness in both legs. Tenderness of the whole trunk including the abdomen, chest, and lower back was observed. Bruising and mild swelling were observed on both legs. Blood tests indicated several abnormalities, including elevated levels of creatine kinase (CK) (669 IU/L; normal range [NR], 0–207 IU/L), aspartate transaminase (AST) (542 IU/L; NR, 8–34 IU/L), and alanine transferase (ALT) (417 IU/L; NR, 9–37 IU/L). Chest and abdomen–pelvis computed tomography (CT) did not detect any evidence of internal organ injury. Brain CT, cervical, thoracic, and lumbar spine CT, and lumbosacral spine magnetic resonance imaging (MRI) were performed to evaluate the weakness of both legs; however, no remarkable findings were found.
After admission, her consciousness improved within several hours, but some laboratory markers followed serially peaked within three days: CK 16,519 IU/L, AST 4,478 IU/L, and ALT 3,978 IU/L. Supportive care consisting of intravenous hydration was maintained. To manage the bilateral whole-leg numbness and stinging symptoms, gabapentin, clonazepam, and subsequent oral prednisolone were used. Because of pain and mild swelling in her left leg, compartment syndrome and blood vessel occlusion were suspected. However, her pedal pulsation was intact, and duplex sonography revealed negative results. She was transferred to a rehabilitation unit 10 days after admission for further evaluation and rehabilitation.
At the time of transfer, initial neurological examination showed muscle strength of hip flexion of 5/4, hip extension of 5/4, knee flexion of 5/2, ankle dorsiflexion of 1/1, big toe extension of 0/1, and ankle plantar flexion of 4/2 by Medical Research Council (MRC) grade on her right/left sides, respectively. She had decreased sensations over the dorsum of the bilateral foot extending into the lateral calf, with tingling sensations on the left sole. Tinel’s sign was observed at the right fibular head. Based on our physical examination, our first impression of the weakness was bilateral sciatic neuropathy.
A nerve conduction study (NCS) and needle electromyography (EMG) were performed 19 days after the trauma (
Tables 1 and
2). The sensory NCS showed a reduced amplitude of sensory nerve action potential in the right superficial peroneal. The motor NCS indicated a decreased compound muscle action potential (CMAP) amplitude with conduction block and segmental slowing around the fibular head in the right common peroneal. Needle EMG demonstrated abnormal spontaneous activity in both biceps femoris (short head), the tibialis anterior, peroneus longus, extensor hallucis longus, and left gastrocnemius with diminished interference patterns. Consequently, right common peroneal and left sciatic neuropathy were strongly suggested, but right sciatic neuropathy could not be excluded because of the abnormal findings in the right biceps femoris (short head).
Table 1
Results of the nerve conduction study
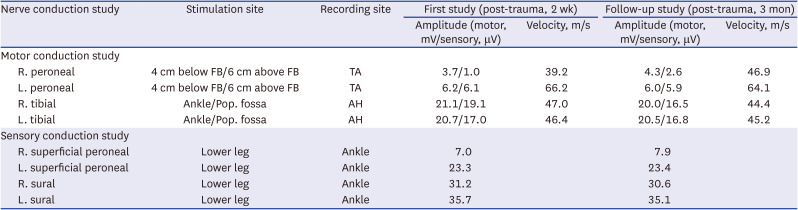
|
Nerve conduction study |
Stimulation site |
Recording site |
First study (post-trauma, 2 wk) |
Follow-up study (post-trauma, 3 mon) |
|
Amplitude (motor, mV/sensory, μV) |
Velocity, m/s |
Amplitude (motor, mV/sensory, μV) |
Velocity, m/s |
|
Motor conduction study |
|
|
|
|
|
|
|
R. peroneal |
4 cm below FB/6 cm above FB |
TA |
3.7/1.0 |
39.2 |
4.3/2.6 |
46.9 |
|
L. peroneal |
4 cm below FB/6 cm above FB |
TA |
6.2/6.1 |
66.2 |
6.0/5.9 |
64.1 |
|
R. tibial |
Ankle/Pop. fossa |
AH |
21.1/19.1 |
47.0 |
20.0/16.5 |
44.4 |
|
L. tibial |
Ankle/Pop. fossa |
AH |
20.7/17.0 |
46.4 |
20.5/16.8 |
45.2 |
|
Sensory conduction study |
|
|
|
|
|
|
|
R. superficial peroneal |
Lower leg |
Ankle |
7.0 |
|
7.9 |
|
|
L. superficial peroneal |
Lower leg |
Ankle |
23.3 |
|
23.4 |
|
|
R. sural |
Lower leg |
Ankle |
31.2 |
|
30.6 |
|
|
L. sural |
Lower leg |
Ankle |
35.7 |
|
35.1 |
|
Table 2
Results of the needle electromyography
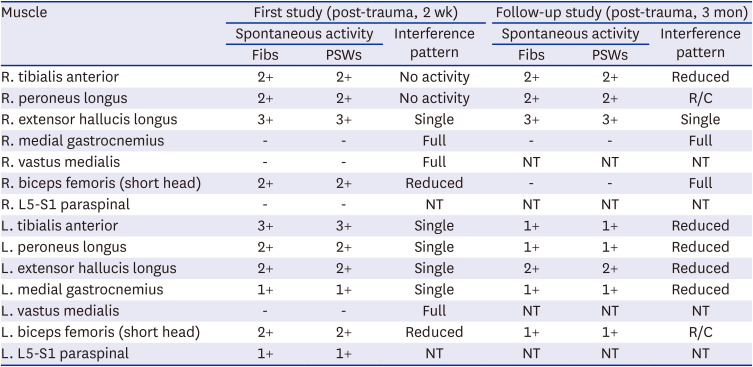
|
Muscle |
First study (post-trauma, 2 wk) |
Follow-up study (post-trauma, 3 mon) |
|
Spontaneous activity |
Interference pattern |
Spontaneous activity |
Interference pattern |
|
Fibs |
PSWs |
Fibs |
PSWs |
|
R. tibialis anterior |
2+ |
2+ |
No activity |
2+ |
2+ |
Reduced |
|
R. peroneus longus |
2+ |
2+ |
No activity |
2+ |
2+ |
R/C |
|
R. extensor hallucis longus |
3+ |
3+ |
Single |
3+ |
3+ |
Single |
|
R. medial gastrocnemius |
- |
- |
Full |
- |
- |
Full |
|
R. vastus medialis |
- |
- |
Full |
NT |
NT |
NT |
|
R. biceps femoris (short head) |
2+ |
2+ |
Reduced |
- |
- |
Full |
|
R. L5-S1 paraspinal |
- |
- |
NT |
NT |
NT |
NT |
|
L. tibialis anterior |
3+ |
3+ |
Single |
1+ |
1+ |
Reduced |
|
L. peroneus longus |
2+ |
2+ |
Single |
1+ |
1+ |
Reduced |
|
L. extensor hallucis longus |
2+ |
2+ |
Single |
2+ |
2+ |
Reduced |
|
L. medial gastrocnemius |
1+ |
1+ |
Single |
1+ |
1+ |
Reduced |
|
L. vastus medialis |
- |
- |
Full |
NT |
NT |
NT |
|
L. biceps femoris (short head) |
2+ |
2+ |
Reduced |
1+ |
1+ |
R/C |
|
L. L5-S1 paraspinal |
1+ |
1+ |
NT |
NT |
NT |
NT |
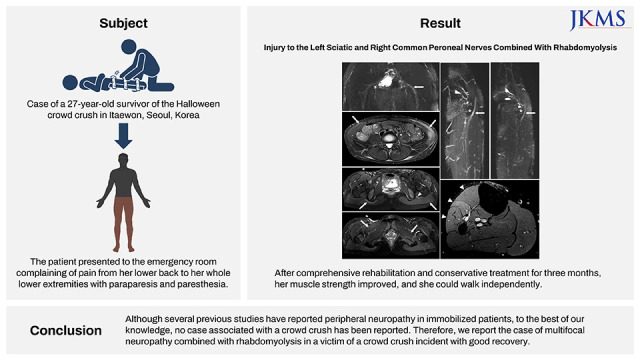
To clarify the diagnosis, the patient underwent MRI of the pelvis and bilateral knee, including magnetic resonance neurography (MRN). The maximum intensity projection (MIP) image revealed swelling of the left sciatic nerve from the sciatic foramen to the proximal thigh (
Fig. 1A). T2-weighted images (T2WI) of the pelvis showed diffuse high signal intensity in the bilateral abdominalis, gluteus maximus, right pectineus, and left quadratus femoris and psoas (
Fig. 1B-D). Furthermore, the knee MIP image showed swelling with enhancement of the right common peroneal nerve, around the fibular head (
Fig. 2A and B). High signal intensities were observed on the T2WI in the right peroneal-innervated muscles (
Fig. 2C). Considering these results, lesions contributing to the weakness were present in the left sciatic nerve exiting the greater sciatic foramen and the right common peroneal nerve around the fibular head.
Fig. 1
Magnetic resonance imaging of the pelvis. (A) Swelling of the left sciatic nerve (white arrow) from the sciatic foramen to the proximal thigh compared with the right sciatic nerve on an maximum intensity projection image from a coronal reconstructed three-dimensional-short tau inversion recovery sampling perfection with application optimized contrasts with variable flip-angle image. (B) Increased signal intensity in the bilateral external/internal oblique abdominis (white arrow) and left psoas muscle (asterisk) on an axial T2-weighted image with TSE fat suppression. (C) Diffuse swelling of the left sciatic nerve (white arrowhead) surrounded by diffuse edema and high signal change in the left gluteal muscle (white arrow) compared with only a focal signal change near the ischial tuberosity of the right gluteal muscle (white arrow) on an axial T2-weighted slice with TSE fat suppression. (D) Increased signal intensity in the right pectineus (white arrow) and left quadratus femoris muscles (white arrow).
TSE = turbo spin echo.
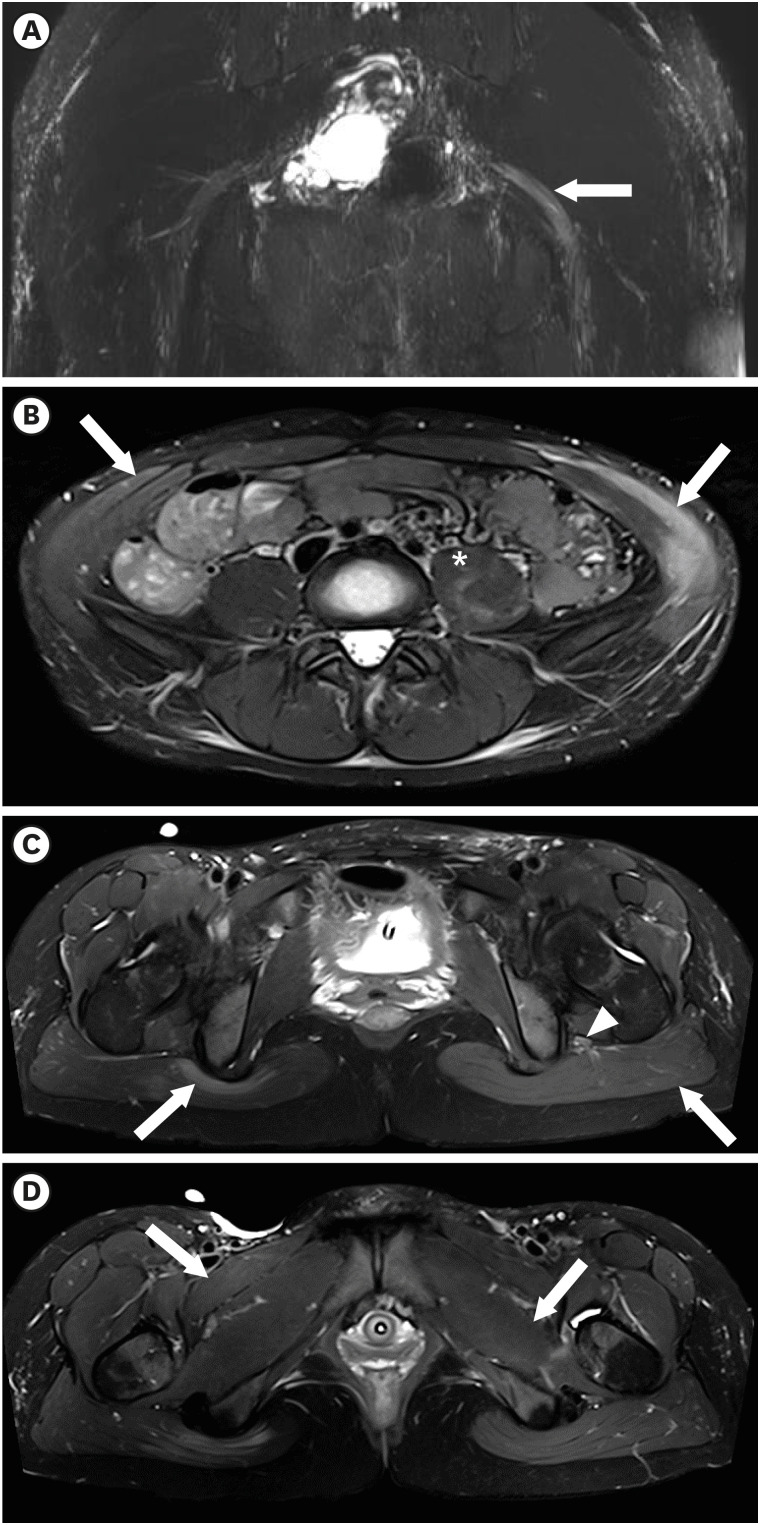
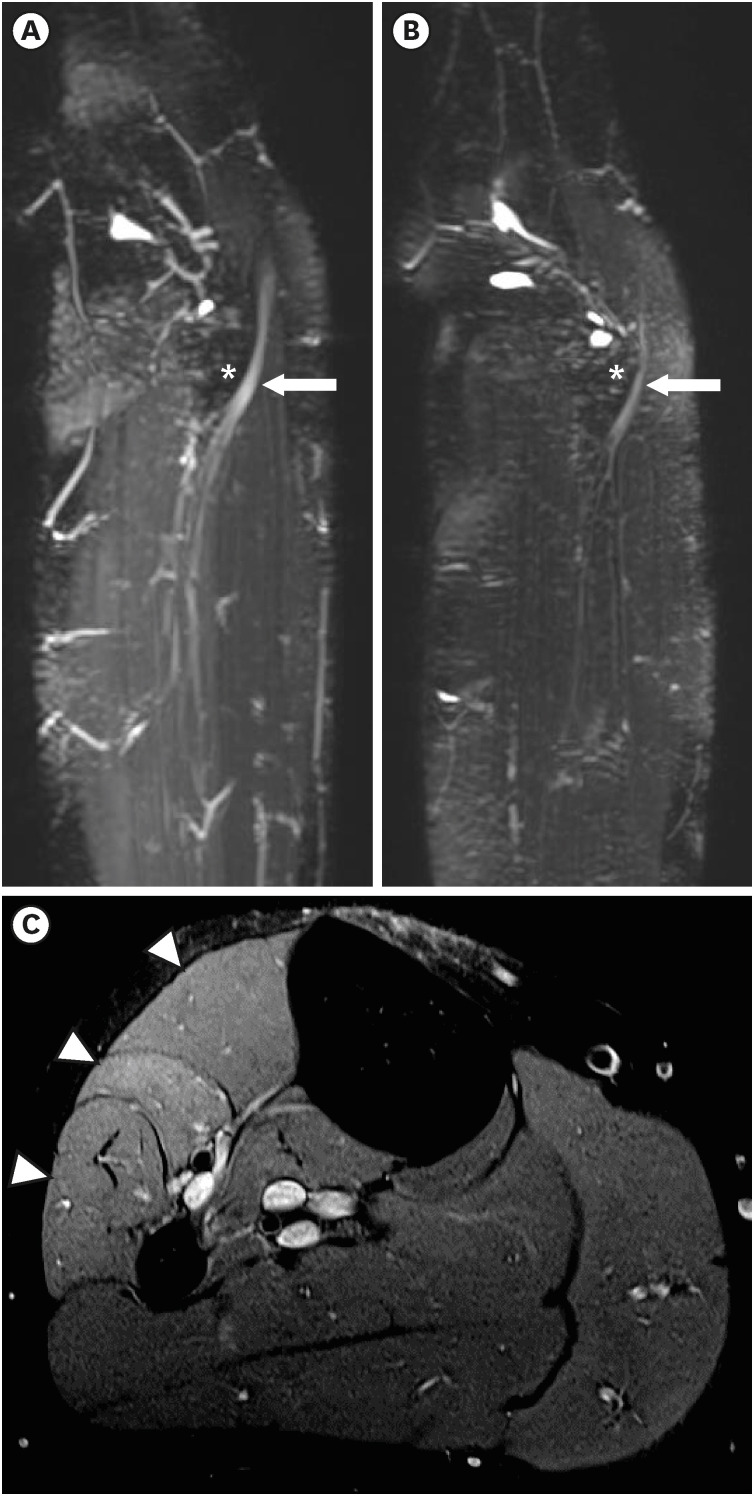
Fig. 2
Magnetic resonance imaging of the knee. (A, B) Swelling with enhancement of the right common peroneal nerve (A, white arrow), which winds around the lateral aspect of the fibular head (asterisk), compared with the left common peroneal nerve (B, white arrow) on an maximum intensity projection image from a coronal-reconstructed three-dimensional short tau inversion recovery sampling perfection with application optimized contrasts with variable flip-angle evolutions image. (C) Ill-defined hyper signal intensity changes in right peroneal-innervated muscles (tibialis anterior, extensor digitorum longus, and peroneal longus) (white arrow heads) compared with no signal change in tibial-innervated muscles on the proton density axial turbo spin echo fat-suppression magnetic resonance image.
Comprehensive rehabilitation was initiated by applying bilateral, custom-made ankle–foot orthoses because of bilateral foot drops. CK, AST, and ALT levels normalized four weeks after the trauma. Three months after the injury, the patient’s muscle strength had recovered markedly: ankle dorsiflexion of 3/4, big toe extension of 2/5, and ankle plantar flexion of 5/4 by MRC grade. Her left foot drop was nearly fully recovered and her right foot drop remained but was subtle. In addition, follow-up electrophysiologic studies demonstrated an improvement of the CMAP amplitude of the right peroneal nerve (
Tables 1 and
2).
Ethics statement
The study was approved by the Institute Review Board of Chung-Ang University Hospital (No. 2212-010-19448). Informed consent was obtained from the patient for the publication of this case report.
DISCUSSION
In this report, we describe a case of left sciatic neuropathy and right common peroneal neuropathy with rhabdomyolysis after a crowd crush. In general, peripheral neuropathy combined with rhabdomyolysis is common and can be explained by several etiologies.
6789 First, neuropathy follows prolonged direct nerve compression.
78 Second, compartment syndrome in injured muscles causes nerve compression damage. Third, muscle damage induces local neuroinflammation. Kim et al.
6 reported peripheral neuropathies adjacent to myositis related to rhabdomyolysis after immobilization due to alcohol intoxication. The hip MRI showed extensive necrotizing myositis on the bilateral pelvic and thigh muscles, and severe inflammatory lesions around the sciatic nerve near the muscles.
In our case, the main etiology of sciatic neuropathy was likely to be direct nerve compression accompanied by neuroinflammation. In the pelvic MRI, a focal signal change of the right gluteal maximus near ischial tuberosity was observed, demonstrating the focal muscular injury, compared with diffuse high signal changes in the left gluteal muscle. The injury of the right gluteus maximus around the bony protuberance might indicate direct compression, but the right sciatic nerve adjacent to the muscle was intact. However, global and diffuse injuries were demonstrated in the left gluteus maximus, which might have caused the neuroinflammation of the left sciatic nerve. Moreover, peroneal neuropathy might have resulted from direct nerve compression. The patient was injured by the crowd crush, which clearly denoted the etiologies of direct compression. Furthermore, direct compression at the fibular head was more plausible due to its anatomical vulnerability.
10 MRN findings of the right knee also represented compression-induced neuropathy. The high signal intensity in the right peroneal-innervated muscles of the T2WI might indicate secondary denervation in the right common peroneal neuropathy (
Fig. 2).
The distinguishing clinical features of our case compared with previous reports are as follows. First, the duration of immobilization was relatively short, but the amount of pressure was extremely high, because of the trauma characteristics. According to a previous case study,
2 more than seven hours of pressure on the buttock was required to generate compressive neuropathy. However, less time was required to cause compressive neuropathy in our case, probably because of the extraordinarily high pressure, which enabled focal neuropathy development. When crushing happens, pressure within the crowd is high enough to bend steel barriers or break brick walls.
11 In terms of compressive neuropathy, both the duration of immobility and the amount of pressure are likely to be important factors. Second, her immobilized position in the crowd crush was uncommon. The patient explained that she fell forward as people pushed on her back. Consequently, she was fixed in a specific posture: prone with a left lateral semi-decubitus position. Uneven pressure on her body, especially concentrated on her left hip and right lateral knee may have led to the compressive neuropathies of her left sciatic and right common peroneal nerves, respectively. Thus, our case with the co-presentation of left sciatic and right common peroneal neuropathies may be related to her peculiar posture in the crowd crush.
There have been a few studies on the prognosis and recovery of patients with neuropathy accompanied by rhabdomyolysis. Previous studies reported that neurologic deficits from neuropathy often remained with incomplete recovery, with varying degrees of improvement at follow-up.
6789 Kim et al.
6 reported a case with multiple peripheral neuropathy combined with rhabdomyolysis, which had a poor prognosis without motor recovery at 6 months, whereas Seok et al.
8 demonstrated that two patients with initial severe ankle dorsiflexion weakness less than 3 on the MRC grade recovered significantly at 6 months. Our case showed a relatively good prognosis, which might be related to the results of NCS showing the relative preservation of the CMAP amplitude of the peroneal and tibial nerves at 3 months follow-up, as well as her young age and the duration of compression. Further research is needed to understand the recovery and prognosis of patients with neuropathy accompanied by rhabdomyolysis.
In clinical practice, it is difficult to localize peripheral nerve lesions based solely on the results of an electrophysiological study. In our case, it was not easy to differentiate whether the cause of the right foot drop was due to a lesion in the common peroneal nerve or the sciatic nerve. In the initial needle EMG, abnormal spontaneous activity was observed in the peroneal-innervated muscles of the lower leg and the biceps femoris (short head), indicating the possibility of a sciatic nerve lesion. However, abnormal spontaneous activity was limited to the peroneal-innervated muscles of the lower leg in the follow-up study, and MRN showed swelling around the fibular head rather than proximal sciatic nerve swelling. Therefore, we considered the possibility of common peroneal neuropathy rather than sciatic neuropathy as the cause of the right foot drop. The abnormal spontaneous activity observed in the right biceps femoris (short head), during the initial needle EMG was considered to be a direct muscle injury. It was also not easy to localize the peripheral nerve lesion that explained the left ankle dorsiflexion and plantarflexion weakness. Although the initial CMAP amplitude of left peroneal and tibial nerves was relatively well preserved, the needle EMG showed abnormal spontaneous activity in the peroneal-innervated muscles and tibial-innervated muscle and lumbar paraspinalis, indicating sciatic nerve lesion or lumbar radiculopathy. However, in the follow-up needle EMG, abnormal spontaneous activity was limited to the peroneal-innervated muscles including the biceps femoris (short head) and tibial-innervated muscles, and MRN showed clear proximal sciatic nerve swelling. Therefore, we considered sciatic neuropathy was the cause of the left ankle weakness. However, we could not exclude the combination of sciatic neuropathy (peroneal portion) with tibial neuropathy because of the lack of sampling tibial-innervated hamstring muscles in needle EMG.
In summary, we report an unusual case of compressive neuropathy combined with rhabdomyolysis caused by a crowd crush. It is important for physicians to localize the compressive neuropathy in patients with rhabdomyolysis and neurologic deficits. Careful physical examination, electrophysiologic and imaging studies, such as MRI or regional MRN, should be performed to make accurate diagnoses.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download