1. Park SH, Im JP, Park H, Jeong SK, Lee JH, Rhee KH, et al. Clinical features and long-term outcomes of paediatric-onset inflammatory bowel disease in a population-based cohort in the Songpa-Kangdong district of Seoul, Korea. J Crohns Colitis. 2022; 16(2):207–215. PMID:
34309652.
2. Lee JW, Eun CS. Inflammatory bowel disease in Korea: epidemiology and pathophysiology. Korean J Intern Med. 2022; 37(5):885–894. PMID:
35902371.
3. Choe JY, Choi S, Song KH, Jang HJ, Choi KH, Yi DY, et al. Incidence and prevalence trends of pediatric inflammatory bowel disease in the Daegu-Kyungpook Province from 2017 to 2020. Front Pediatr. 2022; 9:810173. PMID:
35059365.
4. Park S, Kang B, Kim S, Choi S, Suh HR, Kim ES, et al. Comparison between pediatric Crohn’s disease and ulcerative colitis at diagnosis in Korea: results from a multicenter, registry-based, inception cohort study. Gut Liver. 2022; 16(6):921–929. PMID:
36059091.
5. Choi SY, Choi S, Kang B, Choe BH, Lee YJ, Park JH, et al. Epidemiological trends of pediatric inflammatory bowel disease in Korea: a multicenter study of the last 3 years including the COVID-19 era. J Korean Med Sci. 2022; 37(37):e279. PMID:
36163477.
6. Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr. 2015; 169(11):1053–1060. PMID:
26414706.
7. Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014; 58(6):795–806. PMID:
24231644.
8. Medellin A, Merrill C, Wilson SR. Role of contrast-enhanced ultrasound in evaluation of the bowel. Abdom Radiol (NY). 2018; 43(4):918–933. PMID:
29260278.
9. Zezos P, Zittan E, Islam S, Hudson J, Ben-Bassat O, Nazarian A, et al. Associations between quantitative evaluation of bowel wall microvascular flow by contrast-enhanced ultrasound and indices of disease activity in Crohn’s disease patients using both bolus and infusion techniques. J Clin Ultrasound. 2019; 47(8):453–460. PMID:
31343081.
10. Kljucevsek D, Vidmar D, Urlep D, Dezman R. Dynamic contrast-enhanced ultrasound of the bowel wall with quantitative assessment of Crohn’s disease activity in childhood. Radiol Oncol. 2016; 50(4):347–354. PMID:
27904441.
11. Martínez MJ, Ripollés T, Paredes JM, Moreno-Osset E, Pazos JM, Blanc E. Intravenous contrast-enhanced ultrasound for assessing and grading postoperative recurrence of Crohn’s disease. Dig Dis Sci. 2019; 64(6):1640–1650. PMID:
30604372.
12. Quaia E, Cabibbo B, De Paoli L, Toscano W, Poillucci G, Cova MA. The value of time-intensity curves obtained after microbubble contrast agent injection to discriminate responders from non-responders to anti-inflammatory medication among patients with Crohn’s disease. Eur Radiol. 2013; 23(6):1650–1659. PMID:
23306710.
13. Mudambi K, Sandberg J, Bass D, Rubesova E. Contrast enhanced ultrasound: comparing a novel modality to MRI to assess for bowel disease in pediatric Crohn’s patients. Transl Gastroenterol Hepatol. 2020; 5:13. PMID:
32258517.
14. Benjamin JL, Dennis R, White S Jr, Munson D, Anupindi SA, Piskunowicz M, et al. Improved diagnostic sensitivity of bowel disease of prematurity on contrast-enhanced ultrasound. J Ultrasound Med. 2020; 39(5):1031–1036. PMID:
31705672.
15. Thimm MA, Cuffari C, Garcia A, Sidhu S, Hwang M. Contrast-enhanced ultrasound and shear wave elastography evaluation of Crohn’s disease activity in three adolescent patients. Pediatr Gastroenterol Hepatol Nutr. 2019; 22(3):282–290. PMID:
31110961.
16. Ponorac S, Gošnak RD, Urlep D, Ključevšek D. Contrast-enhanced ultrasonography in the evaluation of Crohn disease activity in children: comparison with histopathology. Pediatr Radiol. 2021; 51(3):410–418. PMID:
33411024.
17. Moskovitz DN, Daperno M, Assche GA. Defining and validating cut-offs for the Simple Endocopic Score for Crohn’s disease. Gastroenterology. 2007; 132:S1097.
18. Sidhu PS, Cantisani V, Deganello A, Dietrich CF, Duran C, Franke D, et al. Role of contrast-enhanced ultrasound (CEUS) in paediatric practice: an EFSUMB position statement. Ultraschall Med. 2017; 38(1):33–43. PMID:
27414980.
19. Piskunowicz M, Kosiak W, Batko T, Piankowski A, Połczyńska K, Adamkiewicz-Drożyńska E. Safety of intravenous application of second-generation ultrasound contrast agent in children: prospective analysis. Ultrasound Med Biol. 2015; 41(4):1095–1099. PMID:
25701526.
20. Migaleddu V, Scanu AM, Quaia E, Rocca PC, Dore MP, Scanu D, et al. Contrast-enhanced ultrasonographic evaluation of inflammatory activity in Crohn’s disease. Gastroenterology. 2009; 137(1):43–52. PMID:
19422826.
21. Girlich C, Jung EM, Huber E, Ott C, Iesalnieks I, Schreyer A, et al. Comparison between preoperative quantitative assessment of bowel wall vascularization by contrast-enhanced ultrasound and operative macroscopic findings and results of histopathological scoring in Crohn’s disease. Ultraschall Med. 2011; 32(2):154–159. PMID:
20449794.
22. Quaia E, De Paoli L, Stocca T, Cabibbo B, Casagrande F, Cova MA. The value of small bowel wall contrast enhancement after sulfur hexafluoride-filled microbubble injection to differentiate inflammatory from fibrotic strictures in patients with Crohn’s disease. Ultrasound Med Biol. 2012; 38(8):1324–1332. PMID:
22698508.
23. Paredes JM, Ripollés T, Cortés X, Moreno N, Martínez MJ, Bustamante-Balén M, et al. Contrast-enhanced ultrasonography: usefulness in the assessment of postoperative recurrence of Crohn’s disease. J Crohn’s Colitis. 2013; 7(3):192–201. PMID:
22542055.
24. Schreyer AG, Finkenzeller T, Gössmann H, Daneschnejad M, Müller-Wille R, Schacherer D, et al. Microcirculation and perfusion with contrast enhanced ultrasound (CEUS) in Crohn’s disease: first results with linear contrast harmonic imaging (CHI). Clin Hemorheol Microcirc. 2008; 40(2):143–155. PMID:
19029639.
25. Liu C, Xu XR, Xu HX, Liu ZJ, Zhang YF, Sun LP, et al. Conventional ultrasound and contrast-enhanced ultrasound in evaluating the severity of Crohn’s disease. Int J Clin Exp Med. 2015; 8(1):123–134. PMID:
25784981.
26. Romanini L, Passamonti M, Navarria M, Lanzarotto F, Villanacci V, Grazioli L, et al. Quantitative analysis of contrast-enhanced ultrasonography of the bowel wall can predict disease activity in inflammatory bowel disease. Eur J Radiol. 2014; 83(8):1317–1323. PMID:
24908589.
27. De Franco A, Di Veronica A, Armuzzi A, Roberto I, Marzo M, De Pascalis B, et al. Ileal Crohn disease: mural microvascularity quantified with contrast-enhanced US correlates with disease activity. Radiology. 2012; 262(2):680–688. PMID:
22157203.
28. Wong DD, Forbes GM, Zelesco M, Mason R, Pawlik J, Mendelson RM. Crohn’s disease activity: quantitative contrast-enhanced ultrasound assessment. Abdom Imaging. 2012; 37(3):369–376. PMID:
21830051.
29. Serafin Z, Białecki M, Białecka A, Sconfienza LM, Kłopocka M. Contrast-enhanced ultrasound for detection of Crohn’s disease activity: systematic review and meta-analysis. J Crohn’s Colitis. 2016; 10(3):354–362. PMID:
26507861.
30. Ripollés T, Rausell N, Paredes JM, Grau E, Martínez MJ, Vizuete J. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn’s disease: a comparison with surgical histopathology analysis. J Crohn’s Colitis. 2013; 7(2):120–128. PMID:
22483566.
31. Turner D, Ricciuto A, Lewis A, D’Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160(5):1570–1583. PMID:
33359090.
32. Lee S, Choi YH, Cho YJ, Cheon JE, Moon JS, Kang GH, et al. Quantitative evaluation of Crohn’s disease using dynamic contrast-enhanced MRI in children and young adults. Eur Radiol. 2020; 30(6):3168–3177. PMID:
32078012.
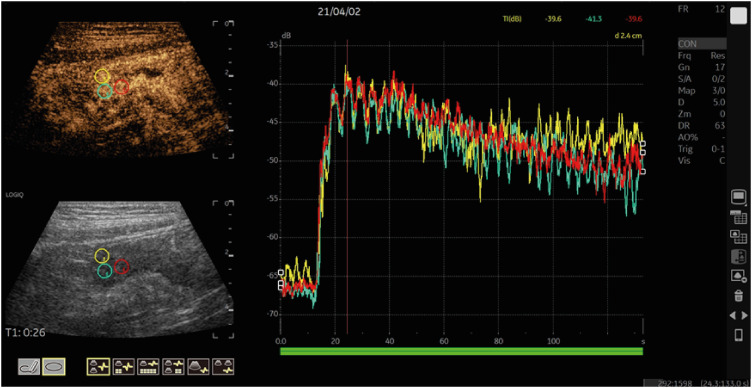
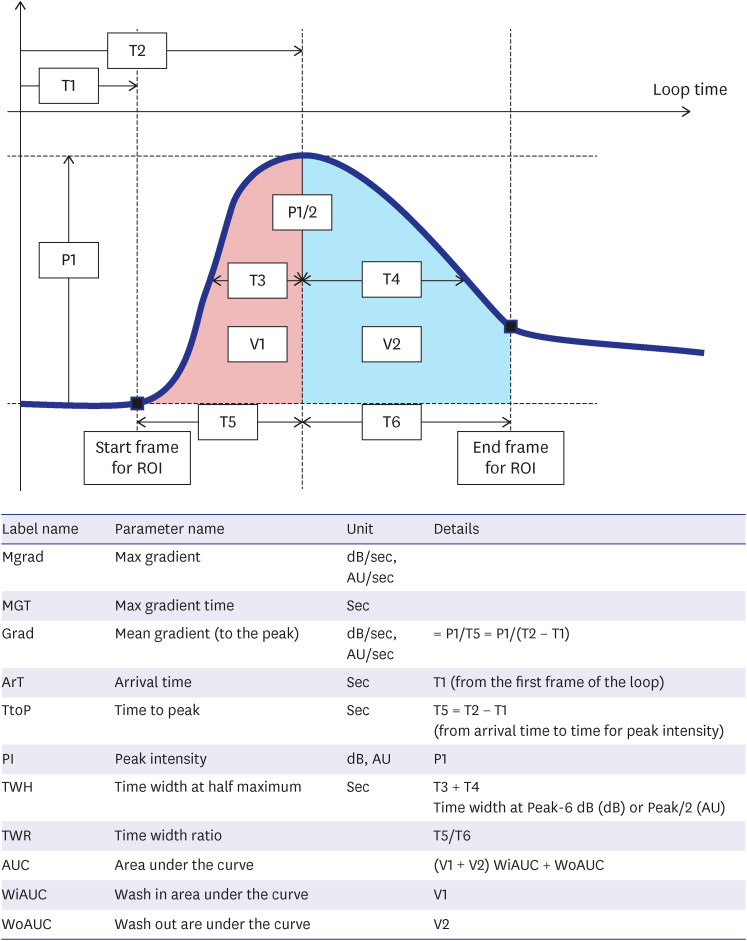
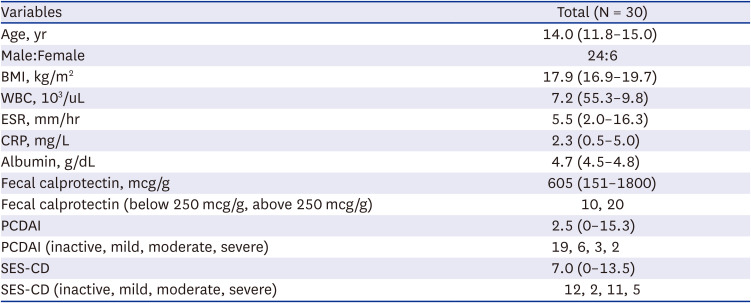
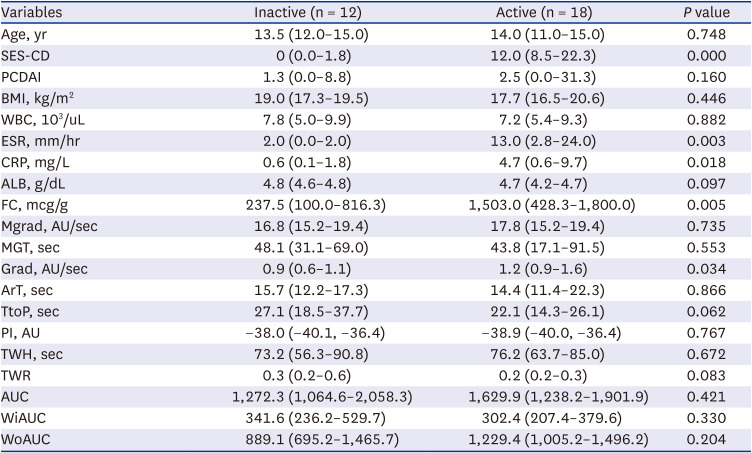





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download